Exploratory Analysis of TP53 Mutations in Circulating Tumour DNA as Biomarkers of Treatment Response for Patients with Relapsed High-Grade Serous Ovarian Carcinoma: A Retrospective Study
- PMID: 27997533
- PMCID: PMC5172526
- DOI: 10.1371/journal.pmed.1002198
Exploratory Analysis of TP53 Mutations in Circulating Tumour DNA as Biomarkers of Treatment Response for Patients with Relapsed High-Grade Serous Ovarian Carcinoma: A Retrospective Study
Abstract
Background: Circulating tumour DNA (ctDNA) carrying tumour-specific sequence alterations may provide a minimally invasive means to dynamically assess tumour burden and response to treatment in cancer patients. Somatic TP53 mutations are a defining feature of high-grade serous ovarian carcinoma (HGSOC). We tested whether these mutations could be used as personalised markers to monitor tumour burden and early changes as a predictor of response and time to progression (TTP).
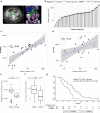
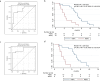
Methods and findings: We performed a retrospective analysis of serial plasma samples collected during routine clinical visits from 40 patients with HGSOC undergoing heterogeneous standard of care treatment. Patient-specific TP53 assays were developed for 31 unique mutations identified in formalin-fixed paraffin-embedded tumour DNA from these patients. These assays were used to quantify ctDNA in 318 plasma samples using microfluidic digital PCR. The TP53 mutant allele fraction (TP53MAF) was compared to serum CA-125, the current gold-standard response marker for HGSOC in blood, as well as to disease volume on computed tomography scans by volumetric analysis. Changes after one cycle of treatment were compared with TTP. The median TP53MAF prior to treatment in 51 relapsed treatment courses was 8% (interquartile range [IQR] 1.2%-22%) compared to 0.7% (IQR 0.3%-2.0%) for seven untreated newly diagnosed stage IIIC/IV patients. TP53MAF correlated with volumetric measurements (Pearson r = 0.59, p < 0.001), and this correlation improved when patients with ascites were excluded (r = 0.82). The ratio of TP53MAF to volume of disease was higher in relapsed patients (0.04% per cm3) than in untreated patients (0.0008% per cm3, p = 0.004). In nearly all relapsed patients with disease volume > 32 cm3, ctDNA was detected at ≥20 amplifiable copies per millilitre of plasma. In 49 treatment courses for relapsed disease, pre-treatment TP53MAF concentration, but not CA-125, was associated with TTP. Response to chemotherapy was seen earlier with ctDNA, with a median time to nadir of 37 d (IQR 28-54) compared with a median time to nadir of 84 d (IQR 42-116) for CA-125. In 32 relapsed treatment courses evaluable for response after one cycle of chemotherapy, a decrease in TP53MAF of >60% was an independent predictor of TTP in multivariable analysis (hazard ratio 0.22, 95% CI 0.07-0.67, p = 0.008). Conversely, a decrease in TP53MAF of ≤60% was associated with poor response and identified cases with TTP < 6 mo with 71% sensitivity (95% CI 42%-92%) and 88% specificity (95% CI 64%-99%). Specificity was improved when patients with recent drainage of ascites were excluded. Ascites drainage led to a reduction of TP53MAF concentration. The limitations of this study include retrospective design, small sample size, and heterogeneity of treatment within the cohort.
Conclusions: In this retrospective study, we demonstrated that ctDNA is correlated with volume of disease at the start of treatment in women with HGSOC and that a decrease of ≤60% in TP53MAF after one cycle of chemotherapy was associated with shorter TTP. These results provide evidence that ctDNA has the potential to be a highly specific early molecular response marker in HGSOC and warrants further investigation in larger cohorts receiving uniform treatment.
Conflict of interest statement
We have read the journal's policy and the authors of this manuscript have the following competing interests: DG, NR and JDB are co-founders, shareholders and officers/consultants of Inivata Ltd, a cancer genomics company that commercialises ctDNA analysis.
Figures


Similar articles
-
Prospective study of the efficacy and utility of TP53 mutations in circulating tumor DNA as a non-invasive biomarker of treatment response monitoring in patients with high-grade serous ovarian carcinoma.J Gynecol Oncol. 2019 May;30(3):e32. doi: 10.3802/jgo.2019.30.e32. J Gynecol Oncol. 2019. PMID: 30887755 Free PMC article.
-
Personalized Circulating Tumor DNA Biomarkers Dynamically Predict Treatment Response and Survival In Gynecologic Cancers.PLoS One. 2015 Dec 30;10(12):e0145754. doi: 10.1371/journal.pone.0145754. eCollection 2015. PLoS One. 2015. PMID: 26717006 Free PMC article.
-
Identification of TP53 mutations in circulating tumour DNA in high grade serous ovarian carcinoma using next generation sequencing technologies.Sci Rep. 2023 Jan 6;13(1):278. doi: 10.1038/s41598-023-27445-2. Sci Rep. 2023. PMID: 36609632 Free PMC article.
-
The feasibility of using mutation detection in ctDNA to assess tumor dynamics.Int J Cancer. 2017 Jun 15;140(12):2642-2647. doi: 10.1002/ijc.30620. Epub 2017 Mar 2. Int J Cancer. 2017. PMID: 28124376 Free PMC article. Review.
-
Circulating tumour DNA (ctDNA) as a liquid biopsy for melanoma.Cancer Lett. 2017 Sep 28;404:62-69. doi: 10.1016/j.canlet.2017.06.030. Epub 2017 Jul 4. Cancer Lett. 2017. PMID: 28687355 Review.
Cited by
-
High-throughput approaches for precision medicine in high-grade serous ovarian cancer.J Hematol Oncol. 2020 Oct 9;13(1):134. doi: 10.1186/s13045-020-00971-6. J Hematol Oncol. 2020. PMID: 33036656 Free PMC article. Review.
-
Role of Circulating Biomarkers in Platinum-Resistant Ovarian Cancer.Int J Mol Sci. 2021 Dec 20;22(24):13650. doi: 10.3390/ijms222413650. Int J Mol Sci. 2021. PMID: 34948446 Free PMC article. Review.
-
Urine TERT promoter mutations-based tumor DNA detection in patients with bladder cancer: A pilot study.Mol Clin Oncol. 2021 Dec;15(6):253. doi: 10.3892/mco.2021.2415. Epub 2021 Oct 8. Mol Clin Oncol. 2021. PMID: 34712485 Free PMC article.
-
Non-invasive Technology Advances in Cancer-A Review of the Advances in the Liquid Biopsy for Endometrial and Ovarian Cancers.Front Digit Health. 2020 Dec 11;2:573010. doi: 10.3389/fdgth.2020.573010. eCollection 2020. Front Digit Health. 2020. PMID: 34713045 Free PMC article. Review.
-
Liquid biopsy in ovarian cancer: advantages and limitations for prognosis and diagnosis.Med Oncol. 2023 Aug 10;40(9):265. doi: 10.1007/s12032-023-02128-0. Med Oncol. 2023. PMID: 37561363 Review.
References
-
- Jacobs I, Oram D, Fairbanks J, Turner J, Frost C, Grudzinskas JG. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br J Obstet Gynaecol. 1990;97(10):922–9. - PubMed
-
- Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brunner N, Chan DW, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008;54(12):e11–79. 10.1373/clinchem.2008.105601 - DOI - PubMed
-
- Soletormos G, Duffy MJ, Othman Abu Hassan S, Verheijen RH, Tholander B, Bast RC Jr, et al. Clinical use of cancer biomarkers in epithelial ovarian cancer: updated guidelines from the European Group on Tumor Markers. Int J Gynecol Cancer. 2016;26(1):43–51. 10.1097/IGC.0000000000000586 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

