Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial
- PMID: 27979383
- PMCID: PMC6886121
- DOI: 10.1016/S0140-6736(16)32517-X
Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial
Erratum in
-
Department of Error.Lancet. 2017 Apr 8;389(10077):e5. doi: 10.1016/S0140-6736(17)30904-2. Lancet. 2017. PMID: 28402831 No abstract available.
Abstract
Background: Atezolizumab is a humanised antiprogrammed death-ligand 1 (PD-L1) monoclonal antibody that inhibits PD-L1 and programmed death-1 (PD-1) and PD-L1 and B7-1 interactions, reinvigorating anticancer immunity. We assessed its efficacy and safety versus docetaxel in previously treated patients with non-small-cell lung cancer.
Methods: We did a randomised, open-label, phase 3 trial (OAK) in 194 academic or community oncology centres in 31 countries. We enrolled patients who had squamous or non-squamous non-small-cell lung cancer, were 18 years or older, had measurable disease per Response Evaluation Criteria in Solid Tumors, and had an Eastern Cooperative Oncology Group performance status of 0 or 1. Patients had received one to two previous cytotoxic chemotherapy regimens (one or more platinum based combination therapies) for stage IIIB or IV non-small-cell lung cancer. Patients with a history of autoimmune disease and those who had received previous treatments with docetaxel, CD137 agonists, anti-CTLA4, or therapies targeting the PD-L1 and PD-1 pathway were excluded. Patients were randomly assigned (1:1) to intravenously receive either atezolizumab 1200 mg or docetaxel 75 mg/m2 every 3 weeks by permuted block randomisation (block size of eight) via an interactive voice or web response system. Coprimary endpoints were overall survival in the intention-to-treat (ITT) and PD-L1-expression population TC1/2/3 or IC1/2/3 (≥1% PD-L1 on tumour cells or tumour-infiltrating immune cells). The primary efficacy analysis was done in the first 850 of 1225 enrolled patients. This study is registered with ClinicalTrials.gov, number NCT02008227.
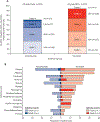
Findings: Between March 11, 2014, and April 29, 2015, 1225 patients were recruited. In the primary population, 425 patients were randomly assigned to receive atezolizumab and 425 patients were assigned to receive docetaxel. Overall survival was significantly longer with atezolizumab in the ITT and PD-L1-expression populations. In the ITT population, overall survival was improved with atezolizumab compared with docetaxel (median overall survival was 13·8 months [95% CI 11·8-15·7] vs 9·6 months [8·6-11·2]; hazard ratio [HR] 0·73 [95% CI 0·62-0·87], p=0·0003). Overall survival in the TC1/2/3 or IC1/2/3 population was improved with atezolizumab (n=241) compared with docetaxel (n=222; median overall survival was 15·7 months [95% CI 12·6-18·0] with atezolizumab vs 10·3 months [8·8-12·0] with docetaxel; HR 0·74 [95% CI 0·58-0·93]; p=0·0102). Patients in the PD-L1 low or undetectable subgroup (TC0 and IC0) also had improved survival with atezolizumab (median overall survival 12·6 months vs 8·9 months; HR 0·75 [95% CI 0·59-0·96]). Overall survival improvement was similar in patients with squamous (HR 0·73 [95% CI 0·54-0·98]; n=112 in the atezolizumab group and n=110 in the docetaxel group) or non-squamous (0·73 [0·60-0·89]; n=313 and n=315) histology. Fewer patients had treatment-related grade 3 or 4 adverse events with atezolizumab (90 [15%] of 609 patients) versus docetaxel (247 [43%] of 578 patients). One treatment-related death from a respiratory tract infection was reported in the docetaxel group.
Interpretation: To our knowledge, OAK is the first randomised phase 3 study to report results of a PD-L1-targeted therapy, with atezolizumab treatment resulting in a clinically relevant improvement of overall survival versus docetaxel in previously treated non-small-cell lung cancer, regardless of PD-L1 expression or histology, with a favourable safety profile.
Funding: F. Hoffmann-La Roche Ltd, Genentech, Inc.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
AR received grants and personal fees from Roche, Eli Lilly, Bristol-Myers Squibb, AstraZeneca, MSD, Boehringer Ingelheim, and Pfizer. FB received personal fees from Roche. DW, MB, and AS are Genentech employees and holders of Roche stock. KP received personal fees and research funding from AstraZeneca, and personal fees from Astellas, AVEO, Boehringer Ingelheim, Clovis, Eli Lilly, Hanmi, KHK Novartis, Ono, and Roche. FC received personal fees from Merck Serono, AstraZeneca, Eli Lilly, Roche, and Bayer. JvP received advisory board fees paid to institution from Daiichi Sankyo, Clovis, Roche, Vertex, and Novartis. SMG received personal fees from Roche/Genentech, Pfizer, Bristol-Myers Squibb, Ariad, Boehringer Ingelheim, and AstraZeneca. TH received grants and personal fees from Chugai, Eli Lilly, Ono, Novartis, Taiho Pharmaceutical, Nippon Boehringer Ingelheim, Pfizer, Bristol-Myers Squibb, Clovis, and AstraZeneca, and grants from Eisai, Takeda, Dainippon Sumitomo Pharma, Abbvie, Merck, Kyowa Hakko Kirin, Daiichi Sankyo, and Astellas. DMK, MCD, DLC, JL, JP, FK, OAF, FDM, HT, J-SL declare no competing interests. CB received clinical research fees from Pfizer, Novartis, Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Roche/Genentech, Eli Lilly, Sanofi, Taiho Pharmaceutical, Mylan, Merrimack, Merck, Abbvie, Astellas, BioMarin, Bristol-Myers Squibb, Daiichi Sankyo, Abraxis BioScience, AB Science, Asana Biosciences, Medivation, Exelixis, ImClone Systems, LEO Pharma, Millennium, and personal fees from Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Roche (Genentech), Eisai, and Bioepis. MK and DSC are Genentech employees, holders of Roche stock, and have a Genentech patent pending for biomarkers and methods treating PD-1-related and PD-L1-related conditions. PH is a Genentech employee, holder of Roche and Amgen stock, and reports that a family member holds Gilead and Allergan stock. DRG received grants for serving as a consultant to Bristol-Myers Squibb, Genentech, and Merck.
Figures



Comment in
-
Programmed death of chemotherapy in non-small-cell lung cancer?Lancet. 2017 Jan 21;389(10066):227-228. doi: 10.1016/S0140-6736(16)32535-1. Epub 2016 Dec 13. Lancet. 2017. PMID: 27979385 No abstract available.
-
Immunotherapy: Benefit with anti-PD-L1.Nat Rev Clin Oncol. 2017 Feb;14(2):70-71. doi: 10.1038/nrclinonc.2016.218. Epub 2016 Dec 29. Nat Rev Clin Oncol. 2017. PMID: 28031557 No abstract available.
-
A mighty oak in the rapidly expanding field of checkpoint inhibition for NSCLC.J Thorac Dis. 2017 Mar;9(3):E292-E294. doi: 10.21037/jtd.2017.02.86. J Thorac Dis. 2017. PMID: 28449524 Free PMC article. No abstract available.
-
Atezolizumab in non-small cell lung cancer: the era of precision immuno-oncology.Ann Transl Med. 2017 Jun;5(12):265. doi: 10.21037/atm.2017.03.89. Ann Transl Med. 2017. PMID: 28706933 Free PMC article. No abstract available.
Similar articles
-
Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial.Lancet. 2016 Apr 30;387(10030):1837-46. doi: 10.1016/S0140-6736(16)00587-0. Epub 2016 Mar 10. Lancet. 2016. PMID: 26970723 Clinical Trial.
-
Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial.Lancet. 2018 Feb 24;391(10122):748-757. doi: 10.1016/S0140-6736(17)33297-X. Epub 2017 Dec 18. Lancet. 2018. PMID: 29268948 Clinical Trial.
-
Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial.Lancet Oncol. 2019 Jul;20(7):924-937. doi: 10.1016/S1470-2045(19)30167-6. Epub 2019 May 20. Lancet Oncol. 2019. PMID: 31122901 Clinical Trial.
-
FDA analyses of survival in older adults with metastatic non-small cell lung cancer in controlled trials of PD-1/PD-L1 blocking antibodies.Semin Oncol. 2018 Aug;45(4):220-225. doi: 10.1053/j.seminoncol.2018.08.007. Epub 2018 Oct 31. Semin Oncol. 2018. PMID: 30391014 Review.
-
New PD-L1 inhibitors in non-small cell lung cancer - impact of atezolizumab.Lung Cancer (Auckl). 2017 Jul 13;8:67-78. doi: 10.2147/LCTT.S113177. eCollection 2017. Lung Cancer (Auckl). 2017. PMID: 28761384 Free PMC article. Review.
Cited by
-
Immune-Related Adverse Events Are Associated With Clinical Benefit in Patients With Non-Small-Cell Lung Cancer Treated With Immunotherapy Plus Chemotherapy: A Retrospective Study.Front Oncol. 2021 Mar 23;11:630136. doi: 10.3389/fonc.2021.630136. eCollection 2021. Front Oncol. 2021. PMID: 33833990 Free PMC article.
-
Safety and efficacy of retreatment with immune checkpoint inhibitors in non-small cell lung cancer: a systematic review and meta-analysis.Transl Lung Cancer Res. 2022 Aug;11(8):1555-1566. doi: 10.21037/tlcr-22-140. Transl Lung Cancer Res. 2022. PMID: 36090645 Free PMC article.
-
Development of canine PD-1/PD-L1 specific monoclonal antibodies and amplification of canine T cell function.PLoS One. 2020 Jul 2;15(7):e0235518. doi: 10.1371/journal.pone.0235518. eCollection 2020. PLoS One. 2020. PMID: 32614928 Free PMC article.
-
Cost-effectiveness analysis of tislelizumab, nivolumab and docetaxel as second- and third-line for advanced or metastatic non-small cell lung cancer in China.Front Pharmacol. 2022 Aug 25;13:880280. doi: 10.3389/fphar.2022.880280. eCollection 2022. Front Pharmacol. 2022. PMID: 36091746 Free PMC article.
-
Novel risk scoring system for immune checkpoint inhibitors treatment in non-small cell lung cancer.Transl Lung Cancer Res. 2021 Feb;10(2):776-789. doi: 10.21037/tlcr-20-832. Transl Lung Cancer Res. 2021. PMID: 33718021 Free PMC article.
References
-
- Stinchcombe TE, Socinski MA. Considerations for second-line therapy of non-small cell lung cancer. Oncologist 2008; 13: 28–36. - PubMed
-
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–50. - PubMed
-
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008; 8: 467–77. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

