Inhibition of cAMP-Dependent PKA Activates β2-Adrenergic Receptor Stimulation of Cytosolic Phospholipase A2 via Raf-1/MEK/ERK and IP3-Dependent Ca2+ Signaling in Atrial Myocytes
- PMID: 27977772
- PMCID: PMC5158063
- DOI: 10.1371/journal.pone.0168505
Inhibition of cAMP-Dependent PKA Activates β2-Adrenergic Receptor Stimulation of Cytosolic Phospholipase A2 via Raf-1/MEK/ERK and IP3-Dependent Ca2+ Signaling in Atrial Myocytes
Abstract
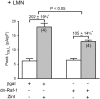
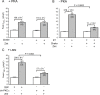
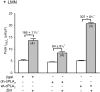
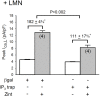
We previously reported in atrial myocytes that inhibition of cAMP-dependent protein kinase (PKA) by laminin (LMN)-integrin signaling activates β2-adrenergic receptor (β2-AR) stimulation of cytosolic phospholipase A2 (cPLA2). The present study sought to determine the signaling mechanisms by which inhibition of PKA activates β2-AR stimulation of cPLA2. We therefore determined the effects of zinterol (0.1 μM; zint-β2-AR) to stimulate ICa,L in atrial myocytes in the absence (+PKA) and presence (-PKA) of the PKA inhibitor (1 μM) KT5720 and compared these results with atrial myocytes attached to laminin (+LMN). Inhibition of Raf-1 (10 μM GW5074), phospholipase C (PLC; 0.5 μM edelfosine), PKC (4 μM chelerythrine) or IP3 receptor (IP3R) signaling (2 μM 2-APB) significantly inhibited zint-β2-AR stimulation of ICa,L in-PKA but not +PKA myocytes. Western blots showed that zint-β2-AR stimulation increased ERK1/2 phosphorylation in-PKA compared to +PKA myocytes. Adenoviral (Adv) expression of dominant negative (dn) -PKCα, dn-Raf-1 or an IP3 affinity trap, each inhibited zint-β2-AR stimulation of ICa,L in + LMN myocytes compared to control +LMN myocytes infected with Adv-βgal. In +LMN myocytes, zint-β2-AR stimulation of ICa,L was enhanced by adenoviral overexpression of wild-type cPLA2 and inhibited by double dn-cPLA2S505A/S515A mutant compared to control +LMN myocytes infected with Adv-βgal. In-PKA myocytes depletion of intracellular Ca2+ stores by 5 μM thapsigargin failed to inhibit zint-β2-AR stimulation of ICa,L via cPLA2. However, disruption of caveolae formation by 10 mM methyl-β-cyclodextrin inhibited zint-β2-AR stimulation of ICa,L in-PKA myocytes significantly more than in +PKA myocytes. We conclude that inhibition of PKA removes inhibition of Raf-1 and thereby allows β2-AR stimulation to act via PKCα/Raf-1/MEK/ERK1/2 and IP3-mediated Ca2+ signaling to stimulate cPLA2 signaling within caveolae. These findings may be relevant to the remodeling of β-AR signaling in failing and/or aging heart, both of which exhibit decreases in adenylate cyclase activity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Laminin enhances beta(2)-adrenergic receptor stimulation of L-type Ca(2+) current via cytosolic phospholipase A(2) signalling in cat atrial myocytes.J Physiol. 2009 Oct 15;587(Pt 20):4785-97. doi: 10.1113/jphysiol.2009.179226. Epub 2009 Aug 24. J Physiol. 2009. PMID: 19703961 Free PMC article.
-
Laminin acts via focal adhesion kinase/phosphatidylinositol-3' kinase/protein kinase B to down-regulate beta1-adrenergic receptor signalling in cat atrial myocytes.J Physiol. 2009 Feb 1;587(3):541-50. doi: 10.1113/jphysiol.2008.163824. Epub 2008 Dec 8. J Physiol. 2009. PMID: 19064616 Free PMC article.
-
The cytosolic phospholipase A2 pathway, a safeguard of beta2-adrenergic cardiac effects in rat.J Biol Chem. 2005 May 13;280(19):18881-90. doi: 10.1074/jbc.M410305200. Epub 2005 Feb 22. J Biol Chem. 2005. PMID: 15728587
-
Postsynaptic localization and regulation of AMPA receptors and Cav1.2 by β2 adrenergic receptor/PKA and Ca2+/CaMKII signaling.EMBO J. 2018 Oct 15;37(20):e99771. doi: 10.15252/embj.201899771. Epub 2018 Sep 24. EMBO J. 2018. PMID: 30249603 Free PMC article. Review.
-
Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation.Biochim Biophys Acta. 2009 Jun;1793(6):959-70. doi: 10.1016/j.bbamcr.2008.12.003. Epub 2008 Dec 16. Biochim Biophys Acta. 2009. PMID: 19133301 Free PMC article. Review.
Cited by
-
Phosphodiesterase 4 is overexpressed in keloid epidermal scars and its inhibition reduces keratinocyte fibrotic alterations.Mol Med. 2024 Sep 2;30(1):134. doi: 10.1186/s10020-024-00906-8. Mol Med. 2024. PMID: 39223490 Free PMC article.
-
Early Protective Role of Inflammation in Cardiac Remodeling and Heart Failure: Focus on TNFα and Resident Macrophages.Cells. 2022 Apr 6;11(7):1249. doi: 10.3390/cells11071249. Cells. 2022. PMID: 35406812 Free PMC article. Review.
-
Impact of Aldosterone on the Failing Myocardium: Insights from Mitochondria and Adrenergic Receptors Signaling and Function.Cells. 2021 Jun 19;10(6):1552. doi: 10.3390/cells10061552. Cells. 2021. PMID: 34205363 Free PMC article. Review.
-
3-Hydroxybutyrate ameliorates insulin resistance by inhibiting PPARγ Ser273 phosphorylation in type 2 diabetic mice.Signal Transduct Target Ther. 2023 May 26;8(1):190. doi: 10.1038/s41392-023-01415-6. Signal Transduct Target Ther. 2023. PMID: 37230992 Free PMC article.
-
Severe Lymphatic Disorder and Multifocal Atrial Tachycardia Treated with Trametinib in a Patient with Noonan Syndrome and SOS1 Mutation.Genes (Basel). 2022 Aug 23;13(9):1503. doi: 10.3390/genes13091503. Genes (Basel). 2022. PMID: 36140671 Free PMC article.
References
-
- Wang YG, Ji X, Pabbidi M, Samarel AM, Lipsius SL. Laminin acts via focal adhesion kinase/phosphatidylinositol-3' kinase/protein kinase B to down-regulate beta1-adrenergic receptor signalling in cat atrial myocytes. The Journal of physiology. 2009;587(3):541–50. Epub 2008/12/10. 19064616; PubMed Central PMCID: PMCPMC2670079. 10.1113/jphysiol.2008.163824 - DOI - PMC - PubMed
-
- Pabbidi MR, Ji X, Samarel AM, Lipsius SL. Laminin enhances beta(2)-adrenergic receptor stimulation of L-type Ca(2+) current via cytosolic phospholipase A(2) signalling in cat atrial myocytes. The Journal of physiology. 2009;587(Pt 20):4785–97. Epub 2009/08/26. PubMed Central PMCID: PMCPMC2770147. 10.1113/jphysiol.2009.179226 - DOI - PMC - PubMed
-
- Pavoine C, Magne S, Sauvadet A, Pecker F. Evidence for a beta2-adrenergic/arachidonic acid pathway in ventricular cardiomyocytes. Regulation by the beta1-adrenergic/camp pathway. The Journal of biological chemistry. 1999;274(2):628–37. Epub 1999/01/05. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

