Mechanism of Protein Kinase R Inhibition by Human Cytomegalovirus pTRS1
- PMID: 27974558
- PMCID: PMC5309967
- DOI: 10.1128/JVI.01574-16
Mechanism of Protein Kinase R Inhibition by Human Cytomegalovirus pTRS1
Abstract
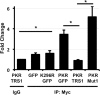
Double-stranded RNAs (dsRNA) produced during human cytomegalovirus (HCMV) infection activate the antiviral kinase protein kinase R (PKR), which potently inhibits virus replication. The HCMV pTRS1 and pIRS1 proteins antagonize PKR to promote HCMV protein synthesis and replication; however, the mechanism by which pTRS1 inhibits PKR is unclear. PKR activation occurs in a three-step cascade. First, binding to dsRNA triggers PKR homodimerizaton. PKR dimers then autophosphorylate, leading to a conformational shift that exposes the binding site for the PKR substrate eIF2α. Consistent with previous in vitro studies, we found that pTRS1 bound and inhibited PKR. pTRS1 binding to PKR was not mediated by an RNA intermediate, and mutations in the pTRS1 RNA binding domain did not affect PKR binding or inhibition. Rather, mutations that disrupted the pTRS1 interaction with PKR ablated the ability of pTRS1 to antagonize PKR activation by dsRNA. pTRS1 did not block PKR dimerization and could bind and inhibit a constitutively dimerized PKR kinase domain. In addition, pTRS1 binding to PKR inhibited PKR kinase activity. Single amino acid point mutations in the conserved eIF2α binding domain of PKR disrupted pTRS1 binding and rendered PKR resistant to inhibition by pTRS1. Consistent with a critical role for the conserved eIF2α contact site in PKR binding, pTRS1 bound an additional eIF2α kinase, heme-regulated inhibitor (HRI), and inhibited eIF2α phosphorylation in response to an HRI agonist. Together our data suggest that pTRS1 inhibits PKR by binding to conserved amino acids in the PKR eIF2α binding site and blocking PKR kinase activity.IMPORTANCE The antiviral kinase PKR plays a critical role in controlling HCMV replication. This study furthered our understanding of how HCMV evades inhibition by PKR and identified new strategies for how PKR activity might be restored during infection to limit HCMV disease.
Keywords: HCMV; PKR; eIF2α; gene expression; human cytomegalovirus; human herpesvirus; innate immunity; mRNA translation; protein kinase R; protein synthesis.
Copyright © 2017 American Society for Microbiology.
Figures






Similar articles
-
Human Cytomegalovirus pTRS1 and pIRS1 Antagonize Protein Kinase R To Facilitate Virus Replication.J Virol. 2016 Mar 28;90(8):3839-3848. doi: 10.1128/JVI.02714-15. Print 2016 Apr. J Virol. 2016. PMID: 26819306 Free PMC article.
-
Protein phosphatase 1 suppresses PKR/EIF2α signaling during human cytomegalovirus infection.J Virol. 2024 Nov 19;98(11):e0059024. doi: 10.1128/jvi.00590-24. Epub 2024 Oct 29. J Virol. 2024. PMID: 39470211
-
Binding and nuclear relocalization of protein kinase R by human cytomegalovirus TRS1.J Virol. 2006 Dec;80(23):11817-26. doi: 10.1128/JVI.00957-06. Epub 2006 Sep 20. J Virol. 2006. PMID: 16987971 Free PMC article.
-
Viral proteins targeting host protein kinase R to evade an innate immune response: a mini review.Biotechnol Genet Eng Rev. 2018 Apr;34(1):33-59. doi: 10.1080/02648725.2018.1467151. Epub 2018 May 2. Biotechnol Genet Eng Rev. 2018. PMID: 29716441 Review.
-
The search for a PKR code-differential regulation of protein kinase R activity by diverse RNA and protein regulators.RNA. 2019 May;25(5):539-556. doi: 10.1261/rna.070169.118. Epub 2019 Feb 15. RNA. 2019. PMID: 30770398 Free PMC article. Review.
Cited by
-
Human cytomegalovirus pTRS1 stimulates cap-independent translation.Virology. 2019 Nov;537:246-253. doi: 10.1016/j.virol.2019.08.026. Epub 2019 Aug 29. Virology. 2019. PMID: 31539772 Free PMC article.
-
Virus-host protein interactions as footprints of human cytomegalovirus replication.Curr Opin Virol. 2022 Feb;52:135-147. doi: 10.1016/j.coviro.2021.11.016. Epub 2021 Dec 16. Curr Opin Virol. 2022. PMID: 34923282 Free PMC article. Review.
-
Multiple functions of stress granules in viral infection at a glance.Front Microbiol. 2023 Mar 1;14:1138864. doi: 10.3389/fmicb.2023.1138864. eCollection 2023. Front Microbiol. 2023. PMID: 36937261 Free PMC article. Review.
-
Minding the message: tactics controlling RNA decay, modification, and translation in virus-infected cells.Genes Dev. 2022 Feb 1;36(3-4):108-132. doi: 10.1101/gad.349276.121. Genes Dev. 2022. PMID: 35193946 Free PMC article. Review.
-
Translational Control in Virus-Infected Cells.Cold Spring Harb Perspect Biol. 2019 Mar 1;11(3):a033001. doi: 10.1101/cshperspect.a033001. Cold Spring Harb Perspect Biol. 2019. PMID: 29891561 Free PMC article. Review.
References
-
- Krishnamoorthy T, Pavitt GD, Zhang F, Dever TE, Hinnebusch AG. 2001. Tight binding of the phosphorylated alpha subunit of initiation factor 2 (eIF2alpha) to the regulatory subunits of guanine nucleotide exchange factor eIF2B is required for inhibition of translation initiation. Mol Cell Biol 21:5018–5030. doi:10.1128/MCB.21.15.5018-5030.2001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

