High-Fat-Diet-Induced Deficits in Dopamine Terminal Function Are Reversed by Restoring Insulin Signaling
- PMID: 27966885
- PMCID: PMC5789793
- DOI: 10.1021/acschemneuro.6b00308
High-Fat-Diet-Induced Deficits in Dopamine Terminal Function Are Reversed by Restoring Insulin Signaling
Abstract
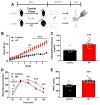
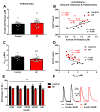
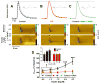
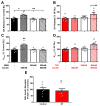
Systemically released insulin crosses the blood-brain barrier and binds to insulin receptors on several neural cell types, including dopaminergic neurons. Insulin has been shown to decrease dopamine neuron firing in the ventral tegmental area (VTA), but potentiate release and reuptake at dopamine terminals in the nucleus accumbens (NAc). Here we show that prolonged consumption of a high fat diet blocks insulin's effects in the NAc, but insulin's effects are restored by inhibiting protein tyrosine phosphatase 1B, which supports insulin receptor signaling. Mice fed a high fat diet (60% kcals from fat) displayed significantly higher fasting blood glucose 160 mg/dL, compared to 101 mg/dL for control-diet-fed mice, and high-fat-diet-fed mice showed reduced blood glucose clearance after an intraperitoneal glucose tolerance test. Using fast scan cyclic voltammetry to measure electrically evoked dopamine in brain slices containing the NAc core, high-fat-diet-fed mice exhibited slower dopamine reuptake compared to control-diet-fed mice (2.2 ± 0.1 and 2.67 ± 0.15 μM/s, respectively). Moreover, glucose clearance rate was negatively correlated with Vmax. Insulin (10 nM to 1 μM) dose dependently increased reuptake rates in control-diet-fed mice compared with in the high-fat-diet group; however, the small molecule insulin receptor sensitizing agent, TCS 401 (300 nM), restored reuptake in high-fat-diet-fed mice to control-diet levels, and a small molecule inhibitor of the insulin receptor, BMS 536924 (300 nM), attenuated reuptake, similar to high-fat-diet-fed mice. These data show that a high-fat diet impairs dopamine reuptake by attenuating insulin signaling at dopamine terminals.
Keywords: DAT; Voltammetry; dopamine; high fat diet; insulin resistance; obesity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Interleukin-6 and tumor necrosis factor-α attenuate dopamine release in mice fed a high-fat diet, but not medium or low-fat diets.Nutr Neurosci. 2023 Sep;26(9):864-874. doi: 10.1080/1028415X.2022.2103613. Epub 2022 Jul 28. Nutr Neurosci. 2023. PMID: 35900193 Free PMC article.
-
Reduced phasic dopamine release and slowed dopamine uptake occur in the nucleus accumbens after a diet high in saturated but not unsaturated fat.Nutr Neurosci. 2022 Jan;25(1):33-45. doi: 10.1080/1028415X.2019.1707421. Epub 2020 Jan 9. Nutr Neurosci. 2022. PMID: 31914869 Free PMC article.
-
Insulin in the ventral tegmental area reduces cocaine-evoked dopamine in the nucleus accumbens in vivo.Eur J Neurosci. 2019 Aug;50(3):2146-2155. doi: 10.1111/ejn.14291. Epub 2018 Dec 11. Eur J Neurosci. 2019. PMID: 30471157
-
The Influence of Strain and Sex on High Fat Diet-Associated Alterations of Dopamine Neurochemistry in Mice.Nutrients. 2024 Sep 29;16(19):3301. doi: 10.3390/nu16193301. Nutrients. 2024. PMID: 39408267 Free PMC article.
-
Obesity and dietary fat influence dopamine neurotransmission: exploring the convergence of metabolic state, physiological stress, and inflammation on dopaminergic control of food intake.Nutr Res Rev. 2022 Dec;35(2):236-251. doi: 10.1017/S0954422421000196. Epub 2021 Jun 28. Nutr Res Rev. 2022. PMID: 34184629 Free PMC article. Review.
Cited by
-
High-fat food biases hypothalamic and mesolimbic expression of consummatory drives.Nat Neurosci. 2020 Oct;23(10):1253-1266. doi: 10.1038/s41593-020-0684-9. Epub 2020 Aug 3. Nat Neurosci. 2020. PMID: 32747789 Free PMC article.
-
Different adaptations of dopamine release in Nucleus Accumbens shell and core of individual alcohol drinking groups of mice.Neuropharmacology. 2020 Sep 15;175:108176. doi: 10.1016/j.neuropharm.2020.108176. Epub 2020 Jun 1. Neuropharmacology. 2020. PMID: 32497591 Free PMC article.
-
Persistent effects of obesity: a neuroplasticity hypothesis.Ann N Y Acad Sci. 2018 Sep;1428(1):221-239. doi: 10.1111/nyas.13665. Epub 2018 May 9. Ann N Y Acad Sci. 2018. PMID: 29741270 Free PMC article. Review.
-
Actions and Consequences of Insulin in the Striatum.Biomolecules. 2023 Mar 11;13(3):518. doi: 10.3390/biom13030518. Biomolecules. 2023. PMID: 36979453 Free PMC article. Review.
-
Nociceptin/orphanin FQ neurons in the Arcuate Nucleus and Ventral Tegmental Area Act via Nociceptin Opioid Peptide Receptor Signaling to Inhibit Proopiomelanocortin and A10 Dopamine Neurons and Thereby Modulate Ingestion of Palatable Food.Physiol Behav. 2021 Jan 1;228:113183. doi: 10.1016/j.physbeh.2020.113183. Epub 2020 Sep 23. Physiol Behav. 2021. PMID: 32979341 Free PMC article.
References
-
- Storlien LH, Pan DA, Kriketos AD, Baur LA. High fat diet-induced insulin resistance. Lessons and implications from animal studies. Ann N Y Acad Sci. 1993;683:82–90. - PubMed
-
- Vinuesa A, Pomilio C, Menafra M, Bonaventura MM, Garay L, Mercogliano MF, Schillaci R, Lux Lantos V, Brites F, Beauquis J, Saravia F. Juvenile exposure to a high fat diet promotes behavioral and limbic alterations in the absence of obesity. Psychoneuroendocrinology. 2016;72:22–33. - PubMed
-
- Heni M, Kullmann S, Preissl H, Fritsche A, Häring HU. Impaired insulin action in the human brain: causes and metabolic consequences. Nat Rev Endocrinol. 2015;11:701–11. - PubMed
-
- Williamson R, McNeilly A, Sutherland C. September 15) Insulin resistance in the brain: An old-age or newage problem? Biochem Pharmacol. 2012;84:737. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

