Optimization of Peptide Vaccines to Induce Robust Antitumor CD4 T-cell Responses
- PMID: 27941004
- PMCID: PMC5221568
- DOI: 10.1158/2326-6066.CIR-16-0194
Optimization of Peptide Vaccines to Induce Robust Antitumor CD4 T-cell Responses
Abstract
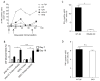
Substantial evidence indicates that immunotherapy is a feasible and effective approach for the treatment of numerous types of cancer. Among various immunotherapy options, peptide vaccines to generate antitumor T cells appear as promising candidates, because of their cost effectiveness and ease of implementation. Nevertheless, most peptide vaccines are notorious for being weekly immunogenic and, thus, optimization of the vaccination strategy is essential to achieve therapeutic effectiveness. In addition, effective peptide vaccines must stimulate both CD8 cytotoxic and CD4 helper T lymphocytes. Our group has been successful in designing effective peptide vaccination strategies for inducing CD8 T-cell responses in mouse tumor models. Here, we describe a somewhat similar, but distinct, peptide vaccination strategy capable of generating vast CD4 T-cell responses by combining synthetic peptides with toll-like receptor (TLR) agonists and OX40/CD40 costimulation. This vaccination strategy was efficient in overcoming immune tolerance to a self-tumor-associated antigen and generated significant antitumor effects in a mouse model of malignant melanoma. The optimized peptide vaccine also allowed the expansion of adoptively transferred CD4 T cells without the need for lymphodepletion and IL2 administration, generating effective antimelanoma responses through the enhancement of proliferative and antiapoptotic activities of CD4 T cells. These results have practical implications in the design of more effective T-cell-based immunotherapies. Cancer Immunol Res; 5(1); 72-83. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
of potential conflicts of interest Esteban Celis has filed patent applications based on the use of synthetic peptides and poly-IC combinatorial vaccines. The rights of the patent applications have been transferred to the Moffitt Cancer Center (Tampa, FL). All other authors declare no conflict of interest.
Figures


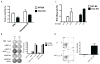

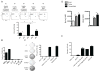
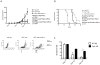
Similar articles
-
Generation of antitumor immunity by cytotoxic T lymphocyte epitope peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4 blockade.Cancer Res. 2003 Jun 15;63(12):3281-8. Cancer Res. 2003. PMID: 12810660
-
In vivo expansion, persistence, and function of peptide vaccine-induced CD8 T cells occur independently of CD4 T cells.Cancer Res. 2008 Dec 1;68(23):9892-9. doi: 10.1158/0008-5472.CAN-08-3134. Cancer Res. 2008. PMID: 19047170 Free PMC article.
-
CD4-directed peptide vaccination augments an antitumor response, but efficacy is limited by the number of CD8+ T cell precursors.J Immunol. 2004 Apr 1;172(7):4215-24. doi: 10.4049/jimmunol.172.7.4215. J Immunol. 2004. PMID: 15034034
-
T-cell epitope peptide vaccines.Expert Rev Vaccines. 2004 Oct;3(5):563-75. doi: 10.1586/14760584.3.5.563. Expert Rev Vaccines. 2004. PMID: 15485336 Review.
-
Peptide-based vaccines for cancer therapy.Hum Vaccin Immunother. 2014;10(11):3175-8. doi: 10.4161/hv.29418. Hum Vaccin Immunother. 2014. PMID: 25483658 Free PMC article. Review.
Cited by
-
Toll-like receptor-targeted anti-tumor therapies: Advances and challenges.Front Immunol. 2022 Nov 21;13:1049340. doi: 10.3389/fimmu.2022.1049340. eCollection 2022. Front Immunol. 2022. PMID: 36479129 Free PMC article. Review.
-
Development of Cancer Immunotherapies.Cancer Treat Res. 2022;183:1-48. doi: 10.1007/978-3-030-96376-7_1. Cancer Treat Res. 2022. PMID: 35551655
-
Cancer immunotherapy: moving forward with peptide T cell vaccines.Curr Opin Immunol. 2017 Aug;47:57-63. doi: 10.1016/j.coi.2017.07.003. Epub 2017 Jul 19. Curr Opin Immunol. 2017. PMID: 28734176 Free PMC article. Review.
-
Potential association factors for developing effective peptide-based cancer vaccines.Front Immunol. 2022 Jul 27;13:931612. doi: 10.3389/fimmu.2022.931612. eCollection 2022. Front Immunol. 2022. PMID: 35967400 Free PMC article.
-
Expression of placenta-specific 1 and its potential for eliciting anti-tumor helper T-cell responses in head and neck squamous cell carcinoma.Oncoimmunology. 2020 Dec 29;10(1):1856545. doi: 10.1080/2162402X.2020.1856545. Oncoimmunology. 2020. PMID: 33457076 Free PMC article.
References
-
- Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation eithout causing graft-versus-host disease. J Clin Oncol. 2016;34:1112–21. - PMC - PubMed
-
- Andersen R, Donia M, Ellebaek E, Borch TH, Kongsted P, Iversen TZ, et al. Long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated IL-2 regimen. Clin Cancer Res. 2016;22:3734–45. - PubMed
-
- Koido S, Homma S, Okamoto M, Takakura K, Mori M, Yoshizaki S, et al. Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms’ tumor 1 (WT1)-specific MHC class I/II-restricted epitopes for pancreatic cancer. Clin Cancer Res. 2014;20:4228–39. - PubMed
-
- Zhang S, Li W, Xia Z, Mao Y. CD4 T cell dependent tumor immunity stimulated by dendritic cell based vaccine. Biochem Biophys Res Commun. 2011;413:294–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

