Normal Wound Healing and Tumor Angiogenesis as a Game of Competitive Inhibition
- PMID: 27935954
- PMCID: PMC5147849
- DOI: 10.1371/journal.pone.0166655
Normal Wound Healing and Tumor Angiogenesis as a Game of Competitive Inhibition
Abstract
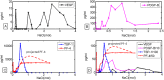
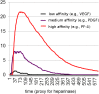
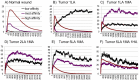
Both normal wound healing and tumor angiogenesis are mitigated by the sequential, carefully orchestrated release of growth stimulators and inhibitors. These regulators are released from platelet clots formed at the sites of activated endothelium in a temporally and spatially controlled manner, and the order of their release depends on their affinity to glycosaminoglycans (GAG) such as heparan sulfate (HS) within the extracellular matrix, and platelet open canallicular system. The formation of vessel sprouts, triggered by angiogenesis regulating factors with lowest affinities for heparan sulfate (e.g. VEGF), is followed by vessel-stabilizing PDGF-B or bFGF with medium affinity for HS, and by inhibitors such as PF-4 and TSP-1 with the highest affinities for HS. The invasive wound-like edge of growing tumors has an overabundance of angiogenesis stimulators, and we propose that their abundance out-competes angiogenesis inhibitors, effectively preventing inhibition of angiogenesis and vessel maturation. We evaluate this hypothesis using an experimentally motivated agent-based model, and propose a general theoretical framework for understanding mechanistic similarities and differences between the processes of normal wound healing and pathological angiogenesis from the point of view of competitive inhibition.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Expression of angiogenesis regulatory proteins and epithelial-mesenchymal transition factors in platelets of the breast cancer patients.ScientificWorldJournal. 2014;2014:878209. doi: 10.1155/2014/878209. Epub 2014 Oct 14. ScientificWorldJournal. 2014. PMID: 25379550 Free PMC article.
-
LHT7, a chemically modified heparin, inhibits multiple stages of angiogenesis by blocking VEGF, FGF2 and PDGF-B signaling pathways.Biomaterials. 2015 Jan;37:271-8. doi: 10.1016/j.biomaterials.2014.10.004. Epub 2014 Oct 28. Biomaterials. 2015. PMID: 25453957
-
Differential role of platelet granular mediators in angiogenesis.Cardiovasc Res. 2004 Aug 1;63(2):226-35. doi: 10.1016/j.cardiores.2004.04.012. Cardiovasc Res. 2004. PMID: 15249180
-
Interactions of platelet factor 4 with the vessel wall.Semin Thromb Hemost. 2004 Jun;30(3):351-8. doi: 10.1055/s-2004-831048. Semin Thromb Hemost. 2004. PMID: 15282658 Review.
-
Antagonising angiogenesis in rheumatoid arthritis.Ann Rheum Dis. 2001 Nov;60 Suppl 3(Suppl 3):iii71-4. doi: 10.1136/ard.60.90003.iii71. Ann Rheum Dis. 2001. PMID: 11890660 Free PMC article. Review. No abstract available.
Cited by
-
Agent-based modeling in cancer biomedicine: applications and tools for calibration and validation.Cancer Biol Ther. 2024 Dec 31;25(1):2344600. doi: 10.1080/15384047.2024.2344600. Epub 2024 Apr 28. Cancer Biol Ther. 2024. PMID: 38678381 Free PMC article. Review.
-
Bioactive Dressing: A New Algorithm in Wound Healing.J Clin Med. 2024 Apr 24;13(9):2488. doi: 10.3390/jcm13092488. J Clin Med. 2024. PMID: 38731023 Free PMC article. Review.
-
Metronomic chemotherapy and drug repurposing: A paradigm shift in oncology.Heliyon. 2024 Jan 14;10(3):e24670. doi: 10.1016/j.heliyon.2024.e24670. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38314272 Free PMC article. Review.
-
Paxillin family of focal adhesion adaptor proteins and regulation of cancer cell invasion.Int Rev Cell Mol Biol. 2020;355:1-52. doi: 10.1016/bs.ircmb.2020.05.003. Epub 2020 Aug 6. Int Rev Cell Mol Biol. 2020. PMID: 32859368 Free PMC article.
-
Co-Expression of VEGF and IL-6 Family Cytokines is Associated with Decreased Survival in HER2 Negative Breast Cancer Patients: Subtype-Specific IL-6 Family Cytokine-Mediated VEGF Secretion.Transl Oncol. 2019 Feb;12(2):245-255. doi: 10.1016/j.tranon.2018.10.004. Epub 2018 Nov 12. Transl Oncol. 2019. PMID: 30439625 Free PMC article.
References
-
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. The New England journal of medicine. 1986;315(26):1650–9. Epub 1986/12/25. - PubMed
-
- Italiano JE Jr., Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111(3):1227–33. 10.1182/blood-2007-09-113837 - DOI - PMC - PubMed
-
- Italiano JE Jr., Shivdasani RA. Megakaryocytes and beyond: the birth of platelets. Journal of thrombosis and haemostasis: JTH. 2003;1(6):1174–82. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

