MCPIP1/Regnase-1 Restricts IL-17A- and IL-17C-Dependent Skin Inflammation
- PMID: 27920272
- PMCID: PMC5225040
- DOI: 10.4049/jimmunol.1601551
MCPIP1/Regnase-1 Restricts IL-17A- and IL-17C-Dependent Skin Inflammation
Abstract
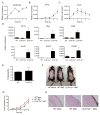
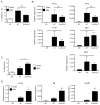
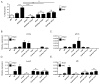
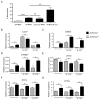
The IL-17 family cytokines IL-17A and IL-17C drive the pathogenesis of psoriatic skin inflammation, and anti-IL-17A Abs were recently approved to treat human psoriasis. Little is known about mechanisms that restrain IL-17 cytokine-mediated signaling, particularly IL-17C. In this article, we show that the endoribonuclease MCP-1-induced protein 1 (MCPIP1; also known as regnase-1) is markedly upregulated in human psoriatic skin lesions. Similarly, MCPIP1 was overexpressed in the imiquimod (IMQ)-driven mouse model of cutaneous inflammation. Mice with an MCPIP1 deficiency (Zc3h12a+/-) displayed no baseline skin inflammation, but they showed exacerbated pathology following IMQ treatment. Pathology in Zc3h12a+/- mice was associated with elevated expression of IL-17A- and IL-17C-dependent genes, as well as with increased accumulation of neutrophils in skin. However, IL-17A and IL-17C expression was unaltered, suggesting that the increased inflammation in Zc3h12a+/- mice was due to enhanced downstream IL-17R signaling. Radiation chimeras demonstrated that MCPIP1 in nonhematopoietic cells is responsible for controlling skin pathology. Moreover, Zc3h12a+/-Il17ra-/- mice given IMQ showed almost no disease. To identify which IL-17RA ligand was essential, Zc3h12a+/-Il17a-/- and Zc3h12a+/-Il17c-/- mice were given IMQ; these mice had reduced but not fully abrogated pathology, indicating that MCPIP1 inhibits IL-17A and IL-17C signaling. Confirming this hypothesis, Zc3h12a-/- keratinocytes showed increased responsiveness to IL-17A and IL-17C stimulation. Thus, MCPIP1 is a potent negative regulator of psoriatic skin inflammation through IL-17A and IL-17C. Moreover, to our knowledge, MCPIP1 is the first described negative regulator of IL-17C signaling.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures

Similar articles
-
MCPIP1 RNase Is Aberrantly Distributed in Psoriatic Epidermis and Rapidly Induced by IL-17A.J Invest Dermatol. 2016 Aug;136(8):1599-1607. doi: 10.1016/j.jid.2016.04.030. Epub 2016 May 12. J Invest Dermatol. 2016. PMID: 27180111
-
Suppression of TCF4 promotes a ZC3H12A-mediated self-sustaining inflammatory feedback cycle involving IL-17RA/IL-17RE epidermal signaling.JCI Insight. 2024 Mar 12;9(8):e172764. doi: 10.1172/jci.insight.172764. JCI Insight. 2024. PMID: 38470486 Free PMC article.
-
MCPIP1 Endoribonuclease Activity Negatively Regulates Interleukin-17-Mediated Signaling and Inflammation.Immunity. 2015 Sep 15;43(3):475-87. doi: 10.1016/j.immuni.2015.07.021. Epub 2015 Aug 25. Immunity. 2015. PMID: 26320658 Free PMC article.
-
The IL-17 Family of Cytokines in Psoriasis: IL-17A and Beyond.Front Immunol. 2018 Aug 2;9:1682. doi: 10.3389/fimmu.2018.01682. eCollection 2018. Front Immunol. 2018. PMID: 30127781 Free PMC article. Review.
-
Regnase-1 Is an Endoribonuclease Essential for the Maintenance of Immune Homeostasis.J Interferon Cytokine Res. 2017 May;37(5):220-229. doi: 10.1089/jir.2017.0001. J Interferon Cytokine Res. 2017. PMID: 28475459 Review.
Cited by
-
Post-transcriptional regulation of immune responses by RNA binding proteins.Proc Jpn Acad Ser B Phys Biol Sci. 2018;94(6):248-258. doi: 10.2183/pjab.94.017. Proc Jpn Acad Ser B Phys Biol Sci. 2018. PMID: 29887569 Free PMC article. Review.
-
IL-17 Signaling: The Yin and the Yang.Trends Immunol. 2017 May;38(5):310-322. doi: 10.1016/j.it.2017.01.006. Epub 2017 Feb 20. Trends Immunol. 2017. PMID: 28254169 Free PMC article. Review.
-
Keratinocyte-specific ablation of Mcpip1 impairs skin integrity and promotes local and systemic inflammation.J Mol Med (Berl). 2019 Dec;97(12):1669-1684. doi: 10.1007/s00109-019-01853-2. Epub 2019 Nov 30. J Mol Med (Berl). 2019. PMID: 31786670 Free PMC article.
-
The RNA binding protein Arid5a drives IL-17-dependent autoantibody-induced glomerulonephritis.J Exp Med. 2024 Sep 2;221(9):e20240656. doi: 10.1084/jem.20240656. Epub 2024 Jul 26. J Exp Med. 2024. PMID: 39058386 Free PMC article.
-
Loss of keratinocyte Mcpip1 abruptly activates the IL-23/Th17 and Stat3 pathways in skin inflammation.Biochim Biophys Acta Mol Cell Res. 2021 Jan;1868(1):118866. doi: 10.1016/j.bbamcr.2020.118866. Epub 2020 Sep 29. Biochim Biophys Acta Mol Cell Res. 2021. PMID: 33007332 Free PMC article. No abstract available.
References
-
- Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B, Rivas E, Tsai TF, Wasel N, Tyring S, Salko T, Hampele I, Notter M, Karpov A, Helou S, Papavassilis C, Group ES, Group FS. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371:326–338. - PubMed
-
- Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH, Durez P, Tak PP, Gomez-Reino JJ, Foster CS, Kim RY, Samson CM, Falk NS, Chu DS, Callanan D, Nguyen QD, Rose K, Haider A, Di Padova F G Psoriasis Study, G Rheumatoid Arthritis Study, G Uveitis Study. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. - PubMed
-
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

