Molecular mechanism of divalent-metal-induced activation of NS3 helicase and insights into Zika virus inhibitor design
- PMID: 27915293
- PMCID: PMC5137455
- DOI: 10.1093/nar/gkw941
Molecular mechanism of divalent-metal-induced activation of NS3 helicase and insights into Zika virus inhibitor design
Abstract
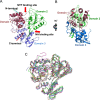
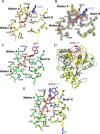
Zika virus has attracted increasing attention because of its potential for causing human neural disorders, including microcephaly in infants and Guillain-Barré syndrome. Its NS3 helicase domain plays critical roles in NTP-dependent RNA unwinding and translocation during viral replication. Our structural analysis revealed a pre-activation state of NS3 helicase in complex with GTPγS, in which the triphosphate adopts a compact conformation in the absence of any divalent metal ions. In contrast, in the presence of a divalent cation, GTPγS adopts an extended conformation, and the Walker A motif undergoes substantial conformational changes. Both features contribute to more extensive interactions between the GTPγS and the enzyme. Thus, this study provides structural evidence on the allosteric modulation of MgNTP2- on the NS3 helicase activity. Furthermore, the compact conformation of inhibitory NTP identified in this study provides precise information for the rational drug design of small molecule inhibitors for the treatment of ZIKV infection.
© The Author(s) 2016. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


Similar articles
-
Structural Insights into the Inhibition of Zika Virus NS2B-NS3 Protease by a Small-Molecule Inhibitor.Structure. 2018 Apr 3;26(4):555-564.e3. doi: 10.1016/j.str.2018.02.005. Epub 2018 Mar 8. Structure. 2018. PMID: 29526431
-
Zika virus NS3 is a canonical RNA helicase stimulated by NS5 RNA polymerase.Nucleic Acids Res. 2019 Sep 19;47(16):8693-8707. doi: 10.1093/nar/gkz650. Nucleic Acids Res. 2019. PMID: 31361901 Free PMC article.
-
Characterization of the Zika virus two-component NS2B-NS3 protease and structure-assisted identification of allosteric small-molecule antagonists.Antiviral Res. 2017 Jul;143:218-229. doi: 10.1016/j.antiviral.2017.04.015. Epub 2017 Apr 29. Antiviral Res. 2017. PMID: 28461069 Free PMC article.
-
Exploiting the unique features of Zika and Dengue proteases for inhibitor design.Biochimie. 2019 Nov;166:132-141. doi: 10.1016/j.biochi.2019.05.004. Epub 2019 May 9. Biochimie. 2019. PMID: 31077760 Review.
-
The Structure of the Zika Virus Protease, NS2B/NS3pro.Adv Exp Med Biol. 2018;1062:131-145. doi: 10.1007/978-981-10-8727-1_10. Adv Exp Med Biol. 2018. PMID: 29845530 Review.
Cited by
-
Crystal structure of the NS3-like helicase from Alongshan virus.IUCrJ. 2020 Apr 10;7(Pt 3):375-382. doi: 10.1107/S2052252520003632. eCollection 2020 May 1. IUCrJ. 2020. PMID: 32431821 Free PMC article.
-
High-throughput crystallographic fragment screening of Zika virus NS3 Helicase.bioRxiv [Preprint]. 2024 Apr 29:2024.04.27.591279. doi: 10.1101/2024.04.27.591279. bioRxiv. 2024. PMID: 38746241 Free PMC article. Preprint.
-
Nucleo-Cytoplasmic Transport of ZIKV Non-Structural 3 Protein Is Mediated by Importin-α/β and Exportin CRM-1.J Virol. 2023 Jan 31;97(1):e0177322. doi: 10.1128/jvi.01773-22. Epub 2022 Dec 8. J Virol. 2023. PMID: 36475764 Free PMC article.
-
Therapeutic Approaches for Zika Virus Infection of the Nervous System.Neurotherapeutics. 2017 Oct;14(4):1027-1048. doi: 10.1007/s13311-017-0575-2. Neurotherapeutics. 2017. PMID: 28952036 Free PMC article. Review.
-
Discovery and Computational Analyses of Novel Small Molecule Zika Virus Inhibitors.Molecules. 2019 Apr 13;24(8):1465. doi: 10.3390/molecules24081465. Molecules. 2019. PMID: 31013906 Free PMC article.
References
-
- Chen L.H., Hamer D.H. Zika virus: rapid spread in the western hemisphere. Ann. Intern. Med. 2016;164:613–615. - PubMed
-
- Triunfol M. A new mosquito-borne threat to pregnant women in Brazil. Lancet Infect. Dis. 2016;16:156–157. - PubMed
-
- Mlakar J., Korva M., Tul N., Popovic M., Poljsak-Prijatelj M., Mraz J., Kolenc M., Resman Rus K., Vesnaver Vipotnik T., Fabjan Vodusek V., et al. Zika virus associated with microcephaly. N. Engl. J. Med. 2016;374:951–958. - PubMed
-
- Li C., Xu D., Ye Q., Hong S., Jiang Y., Liu X., Zhang N., Shi L., Qin C.F., Xu Z. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell. 2016;19:120–126. - PubMed
-
- Roze B., Najioullah F., Ferge J.L., Apetse K., Brouste Y., Cesaire R., Fagour C., Fagour L., Hochedez P., Jeannin S., et al. Zika virus detection in urine from patients with Guillain-Barre syndrome on Martinique, January 2016. Euro Surveill. 2016;21 doi:10.2807/1560-7917.ES.2016.21.9.30154. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

