Distinct cortical and striatal actions of a β-arrestin-biased dopamine D2 receptor ligand reveal unique antipsychotic-like properties
- PMID: 27911814
- PMCID: PMC5167191
- DOI: 10.1073/pnas.1614347113
Distinct cortical and striatal actions of a β-arrestin-biased dopamine D2 receptor ligand reveal unique antipsychotic-like properties
Abstract
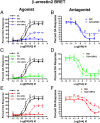
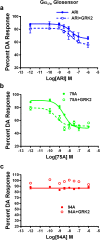
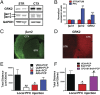
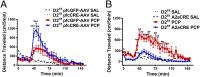
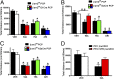
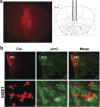
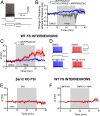
The current dopamine (DA) hypothesis of schizophrenia postulates striatal hyperdopaminergia and cortical hypodopaminergia. Although partial agonists at DA D2 receptors (D2Rs), like aripiprazole, were developed to simultaneously target both phenomena, they do not effectively improve cortical dysfunction. In this study, we investigate the potential for newly developed β-arrestin2 (βarr2)-biased D2R partial agonists to simultaneously target hyper- and hypodopaminergia. Using neuron-specific βarr2-KO mice, we show that the antipsychotic-like effects of a βarr2-biased D2R ligand are driven through both striatal antagonism and cortical agonism of D2R-βarr2 signaling. Furthermore, βarr2-biased D2R agonism enhances firing of cortical fast-spiking interneurons. This enhanced cortical agonism of the biased ligand can be attributed to a lack of G-protein signaling and elevated expression of βarr2 and G protein-coupled receptor (GPCR) kinase 2 in the cortex versus the striatum. Therefore, we propose that βarr2-biased D2R ligands that exert region-selective actions could provide a path to develop more effective antipsychotic therapies.
Keywords: antipsychotics; arrestin; biased signaling; dopamine D2R; fast-spiking interneurons.
Conflict of interest statement
P.O. is an employee and shareholder at Pfizer, Inc. M.G.C. has received compensation from Lundbeck as a member of their Psychopharmacology Advisory Board and is a consultant for Omeros Corp. M.G.C. also owns equity in Acadia Pharmaceuticals.
Figures




Similar articles
-
New Concepts in Dopamine D2 Receptor Biased Signaling and Implications for Schizophrenia Therapy.Biol Psychiatry. 2017 Jan 1;81(1):78-85. doi: 10.1016/j.biopsych.2016.10.011. Epub 2016 Oct 19. Biol Psychiatry. 2017. PMID: 27832841 Free PMC article. Review.
-
Antipsychotic-Like Efficacy of Dopamine D2 Receptor-Biased Ligands is Dependent on Adenosine A2A Receptor Expression.Mol Neurobiol. 2018 Jun;55(6):4952-4958. doi: 10.1007/s12035-017-0696-y. Epub 2017 Aug 5. Mol Neurobiol. 2018. PMID: 28779351
-
D2 Dopamine Receptor G Protein-Biased Partial Agonists Based on Cariprazine.J Med Chem. 2019 May 9;62(9):4755-4771. doi: 10.1021/acs.jmedchem.9b00508. Epub 2019 Apr 18. J Med Chem. 2019. PMID: 30964661 Free PMC article.
-
Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy.Proc Natl Acad Sci U S A. 2011 Nov 8;108(45):18488-93. doi: 10.1073/pnas.1104807108. Epub 2011 Oct 24. Proc Natl Acad Sci U S A. 2011. PMID: 22025698 Free PMC article.
-
Mechanism of new antipsychotic medications: occupancy is not just antagonism.Arch Gen Psychiatry. 2003 Oct;60(10):974-7. doi: 10.1001/archpsyc.60.10.974. Arch Gen Psychiatry. 2003. PMID: 14557141 Review.
Cited by
-
Arrestin-3 Agonism at Dopamine D3 Receptors Defines a Subclass of Second-Generation Antipsychotics That Promotes Drug Tolerance.Biol Psychiatry. 2023 Oct 1;94(7):531-542. doi: 10.1016/j.biopsych.2023.03.006. Epub 2023 Mar 15. Biol Psychiatry. 2023. PMID: 36931452 Free PMC article.
-
Activation of Dopamine 4 Receptor Subtype Enhances Gamma Oscillations in Hippocampal Slices of Aged Mice.Front Aging Neurosci. 2022 Mar 16;14:838803. doi: 10.3389/fnagi.2022.838803. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35370600 Free PMC article.
-
Biased signalling: from simple switches to allosteric microprocessors.Nat Rev Drug Discov. 2018 Apr;17(4):243-260. doi: 10.1038/nrd.2017.229. Epub 2018 Jan 5. Nat Rev Drug Discov. 2018. PMID: 29302067 Free PMC article. Review.
-
Biased Signaling of the Mu Opioid Receptor Revealed in Native Neurons.iScience. 2019 Apr 26;14:47-57. doi: 10.1016/j.isci.2019.03.011. Epub 2019 Mar 15. iScience. 2019. PMID: 30925410 Free PMC article.
-
The Beta-Arrestin-Biased Dopamine D2 Receptor Ligand, UNC9994, Is a Partial Agonist at G-Protein-Mediated Potassium Channel Activation.Int J Neuropsychopharmacol. 2018 Dec 1;21(12):1102-1108. doi: 10.1093/ijnp/pyy059. Int J Neuropsychopharmacol. 2018. PMID: 29986044 Free PMC article.
References
-
- Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1(9):727–730. - PubMed
-
- Allen JA, Roth BL. Strategies to discover unexpected targets for drugs active at G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2011;51:117–144. - PubMed
-
- Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: A protein that regulates beta-adrenergic receptor function. Science. 1990;248(4962):1547–1550. - PubMed
-
- Ferguson SS, et al. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271(5247):363–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

