Cellular Aging Contributes to Failure of Cold-Induced Beige Adipocyte Formation in Old Mice and Humans
- PMID: 27889388
- PMCID: PMC5226893
- DOI: 10.1016/j.cmet.2016.10.023
Cellular Aging Contributes to Failure of Cold-Induced Beige Adipocyte Formation in Old Mice and Humans
Erratum in
-
Cellular Aging Contributes to Failure of Cold-Induced Beige Adipocyte Formation in Old Mice and Humans.Cell Metab. 2017 Feb 7;25(2):481. doi: 10.1016/j.cmet.2017.01.011. Cell Metab. 2017. PMID: 28178569 No abstract available.
Abstract
Cold temperatures induce progenitor cells within white adipose tissue to form beige adipocytes that burn energy and generate heat; this is a potential anti-diabesity therapy. However, the potential to form cold-induced beige adipocytes declines with age. This creates a clinical roadblock to potential therapeutic use in older individuals, who constitute a large percentage of the obesity epidemic. Here we show that aging murine and human beige progenitor cells display a cellular aging, senescence-like phenotype that accounts for their age-dependent failure. Activating the senescence pathway, either genetically or pharmacologically, in young beige progenitors induces premature cellular senescence and blocks their potential to form cold-induced beige adipocytes. Conversely, genetically or pharmacologically reversing cellular aging by targeting the p38/MAPK-p16Ink4a pathway in aged mouse or human beige progenitor cells rejuvenates cold-induced beiging. This in turn increases glucose sensitivity. Collectively, these data indicate that anti-aging or senescence modalities could be a strategy to induce beiging, thereby improving metabolic health in aging humans.
Keywords: Ink4a/Arf; adipose; beige adipocytes; cellular aging; cold exposure; mural cells; senescence; thermogenesis.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
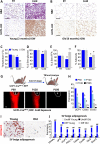
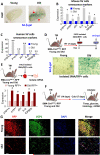
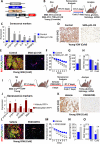
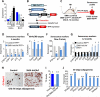
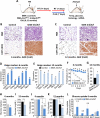
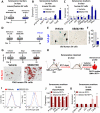
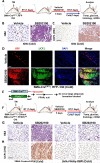
Comment in
-
Adipose tissue: Reversing age-related decline in beiging.Nat Rev Endocrinol. 2017 Feb;13(2):64. doi: 10.1038/nrendo.2016.208. Epub 2016 Dec 9. Nat Rev Endocrinol. 2017. PMID: 27934865 No abstract available.
-
Young and Lean: Elimination of Senescent Cells Boosts Adaptive Thermogenesis.Cell Metab. 2017 Feb 7;25(2):226-228. doi: 10.1016/j.cmet.2017.01.012. Cell Metab. 2017. PMID: 28178562
Similar articles
-
Transient p53 inhibition sensitizes aged white adipose tissue for beige adipocyte recruitment by blocking mitophagy.FASEB J. 2019 Jan;33(1):844-856. doi: 10.1096/fj.201800577R. Epub 2018 Jul 27. FASEB J. 2019. PMID: 30052487 Free PMC article.
-
Aging impairs beige adipocyte differentiation of mesenchymal stem cells via the reduced expression of Sirtuin 1.Biochem Biophys Res Commun. 2018 Jun 7;500(3):682-690. doi: 10.1016/j.bbrc.2018.04.136. Biochem Biophys Res Commun. 2018. PMID: 29678576
-
Aging impairs cold-induced beige adipogenesis and adipocyte metabolic reprogramming.Elife. 2024 May 22;12:RP87756. doi: 10.7554/eLife.87756. Elife. 2024. PMID: 38775132 Free PMC article.
-
Lsd1, a metabolic sensor of environment requirements that prevents adipose tissue from aging.Adipocyte. 2017 Oct 2;6(4):298-303. doi: 10.1080/21623945.2017.1345831. Epub 2017 Jul 12. Adipocyte. 2017. PMID: 28700271 Free PMC article. Review.
-
Epigenetic regulation of beige adipocyte fate by histone methylation.Endocr J. 2019 Feb 28;66(2):115-125. doi: 10.1507/endocrj.EJ18-0442. Epub 2018 Dec 28. Endocr J. 2019. PMID: 30606913 Review.
Cited by
-
The role of brown adipose tissue in mediating healthful longevity.J Cardiovasc Aging. 2024 Apr;4(2):17. doi: 10.20517/jca.2024.01. Epub 2024 Apr 27. J Cardiovasc Aging. 2024. PMID: 39119146 Free PMC article.
-
Stem Cell-Derived Exosomes Prevent Aging-Induced Cardiac Dysfunction through a Novel Exosome/lncRNA MALAT1/NF-κB/TNF-α Signaling Pathway.Oxid Med Cell Longev. 2019 Apr 8;2019:9739258. doi: 10.1155/2019/9739258. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31089420 Free PMC article.
-
Reversing Pdgfrβ Signaling Restores Metabolically Active Beige Adipocytes by Alleviating ILC2 Suppression in Aged and Obese Mice.bioRxiv [Preprint]. 2024 Jun 18:2024.06.17.599436. doi: 10.1101/2024.06.17.599436. bioRxiv. 2024. Update in: Mol Metab. 2024 Nov;89:102028. doi: 10.1016/j.molmet.2024.102028. PMID: 38948810 Free PMC article. Updated. Preprint.
-
Emerging Roles for the INK4a/ARF (CDKN2A) Locus in Adipose Tissue: Implications for Obesity and Type 2 Diabetes.Biomolecules. 2020 Sep 22;10(9):1350. doi: 10.3390/biom10091350. Biomolecules. 2020. PMID: 32971832 Free PMC article. Review.
-
T Cells in Adipose Tissue in Aging.Front Immunol. 2018 Dec 12;9:2945. doi: 10.3389/fimmu.2018.02945. eCollection 2018. Front Immunol. 2018. PMID: 30619305 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

