Optimization of cGMP purification and expansion of umbilical cord blood-derived T-regulatory cells in support of first-in-human clinical trials
- PMID: 27887864
- PMCID: PMC5237605
- DOI: 10.1016/j.jcyt.2016.10.011
Optimization of cGMP purification and expansion of umbilical cord blood-derived T-regulatory cells in support of first-in-human clinical trials
Abstract
Background aims: Thymic-derived regulatory T cells (tTreg) are critical regulators of the immune system. Adoptive tTreg transfer is a curative therapy for murine models of autoimmunity, graft rejection, and graft-versus-host disease (GVHD). We previously completed a "first-in-human" clinical trial using in vitro expanded umbilical cord blood (UCB)-derived tTreg to prevent GVHD in patients undergoing UCB hematopoietic stem cell transplantation (HSCT). tTreg were safe and demonstrated clinical efficacy, but low yield prevented further dose escalation.
Methods: To optimize yield, we investigated the use of KT64/86 artificial antigen presenting cells (aAPCs) to expand tTreg and incorporated a single re-stimulation after day 12 in expansion culture.
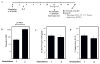
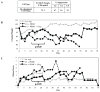
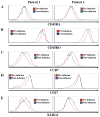
Results: aAPCs increased UCB tTreg expansion greater than eightfold over CD3/28 stimulation. Re-stimulation with aAPCs increased UCB tTreg expansion an additional 20- to 30-fold. Re-stimulated human UCB tTreg ameliorated GVHD disease in a xenogeneic model. Following current Good Manufacturing Practice (cGMP) validation, a trial was conducted with tTreg. tTreg doses up to >30-fold higher compared with that obtained with anti-CD3/28 mAb coated-bead expansion and Foxp3 expression was stable during in vitro expansion and following transfer to patients. Increased expansion did not result in a senescent phenotype and GVHD was significantly reduced.
Discussion: Expansion culture with cGMP aAPCs and re-stimulation reproducibly generates sufficient numbers of UCB tTreg that exceeds the numbers of T effector cells in an UCB graft. The methodology supports future tTreg banking and is adaptable to tTreg expansion from HSC sources. Furthermore, because human leukocyte antigen matching is not required, allogeneic UCB tTreg may be a useful strategy for prevention of organ rejection and autoimmune disease.
Keywords: cGMP production; graft versus host disease; regulatory T cell.
Copyright © 2017 International Society for Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors share a patent entitled “Methods to expand a T Regulatory Cell Master Cell Bank”; US Patent # 13/639,927.
Figures




Similar articles
-
Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells.Blood. 2008 Oct 1;112(7):2847-57. doi: 10.1182/blood-2008-01-132951. Epub 2008 Jul 21. Blood. 2008. PMID: 18645038 Free PMC article.
-
Protective role of functionalized single walled carbon nanotubes enhance ex vivo expansion of hematopoietic stem and progenitor cells in human umbilical cord blood.Nanomedicine. 2013 Nov;9(8):1304-16. doi: 10.1016/j.nano.2013.05.009. Epub 2013 Jun 1. Nanomedicine. 2013. PMID: 23732300
-
Immune regulatory cells in umbilical cord blood and their potential roles in transplantation tolerance.Crit Rev Oncol Hematol. 2011 Aug;79(2):112-26. doi: 10.1016/j.critrevonc.2010.07.009. Epub 2010 Aug 19. Crit Rev Oncol Hematol. 2011. PMID: 20727784 Free PMC article. Review.
-
Methods of ex vivo expansion of human cord blood cells: challenges, successes and clinical implications.Expert Rev Hematol. 2016 Mar;9(3):297-314. doi: 10.1586/17474086.2016.1128321. Epub 2016 Jan 21. Expert Rev Hematol. 2016. PMID: 26635058 Review.
-
Umbilical cord blood-derived mesenchymal stromal cells promote myeloid-derived suppressor cell proliferation by secreting HLA-G to reduce acute graft-versus-host disease after hematopoietic stem cell transplantation.Cytotherapy. 2020 Dec;22(12):718-733. doi: 10.1016/j.jcyt.2020.07.008. Epub 2020 Aug 15. Cytotherapy. 2020. PMID: 32811747
Cited by
-
Trafficking and persistence of alloantigen-specific chimeric antigen receptor regulatory T cells in Cynomolgus macaque.Cell Rep Med. 2022 May 17;3(5):100614. doi: 10.1016/j.xcrm.2022.100614. Epub 2022 May 11. Cell Rep Med. 2022. PMID: 35551746 Free PMC article.
-
Methods to manufacture regulatory T cells for cell therapy.Clin Exp Immunol. 2019 Jul;197(1):52-63. doi: 10.1111/cei.13297. Epub 2019 Apr 15. Clin Exp Immunol. 2019. PMID: 30913302 Free PMC article. Review.
-
GMP-Compliant Universal Antigen Presenting Cells (uAPC) Promote the Metabolic Fitness and Antitumor Activity of Armored Cord Blood CAR-NK Cells.Front Immunol. 2021 Feb 26;12:626098. doi: 10.3389/fimmu.2021.626098. eCollection 2021. Front Immunol. 2021. PMID: 33717142 Free PMC article.
-
Foxp3+ regulatory T cell therapy for tolerance in autoimmunity and solid organ transplantation.Front Immunol. 2022 Nov 17;13:1055466. doi: 10.3389/fimmu.2022.1055466. eCollection 2022. Front Immunol. 2022. PMID: 36466912 Free PMC article. Review.
-
Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect.Blood. 2016 Feb 25;127(8):1044-51. doi: 10.1182/blood-2015-06-653667. Epub 2015 Nov 12. Blood. 2016. PMID: 26563133 Free PMC article. Clinical Trial.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

