Conditional Loss of Pten in Myogenic Progenitors Leads to Postnatal Skeletal Muscle Hypertrophy but Age-Dependent Exhaustion of Satellite Cells
- PMID: 27880908
- PMCID: PMC5181649
- DOI: 10.1016/j.celrep.2016.11.002
Conditional Loss of Pten in Myogenic Progenitors Leads to Postnatal Skeletal Muscle Hypertrophy but Age-Dependent Exhaustion of Satellite Cells
Abstract
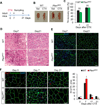
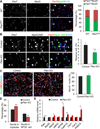
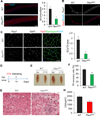
Skeletal muscle stem cells (satellite cells [SCs]) are normally maintained in a quiescent (G0) state. Muscle injury not only activates SCs locally, but also alerts SCs in distant uninjured muscles via circulating factors. The resulting GAlert SCs are adapted to regenerative cues and regenerate injured muscles more efficiently, but whether they provide any long-term benefits to SCs is unknown. Here, we report that embryonic myogenic progenitors lacking the phosphatase and tensin homolog (Pten) exhibit enhanced proliferation and differentiation, resulting in muscle hypertrophy but fewer SCs in adult muscles. Interestingly, Pten null SCs are predominantly in the GAlert state, even in the absence of an injury. The GAlert SCs are deficient in self-renewal and subjected to accelerated depletion during regeneration and aging and fail to repair muscle injury in old mice. Our findings demonstrate a key requirement of Pten in G0 entry of SCs and provide functional evidence that prolonged GAlert leads to stem cell depletion and regenerative failure.
Keywords: Pten; aging; hypertrophy; regeneration; skeletal muscle; stem cells.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Pten is necessary for the quiescence and maintenance of adult muscle stem cells.Nat Commun. 2017 Jan 17;8:14328. doi: 10.1038/ncomms14328. Nat Commun. 2017. PMID: 28094257 Free PMC article.
-
Loss of MyoD and Myf5 in Skeletal Muscle Stem Cells Results in Altered Myogenic Programming and Failed Regeneration.Stem Cell Reports. 2018 Mar 13;10(3):956-969. doi: 10.1016/j.stemcr.2018.01.027. Epub 2018 Mar 1. Stem Cell Reports. 2018. PMID: 29478898 Free PMC article.
-
Transient HIF2A inhibition promotes satellite cell proliferation and muscle regeneration.J Clin Invest. 2018 Jun 1;128(6):2339-2355. doi: 10.1172/JCI96208. Epub 2018 Apr 30. J Clin Invest. 2018. PMID: 29533927 Free PMC article.
-
Aging changes in satellite cells and their functions.Curr Aging Sci. 2011 Dec;4(3):279-97. doi: 10.2174/1874609811104030279. Curr Aging Sci. 2011. PMID: 21529324 Review.
-
Roles of phosphatase and tensin homolog in skeletal muscle.J Cell Physiol. 2019 Apr;234(4):3192-3196. doi: 10.1002/jcp.26820. Epub 2018 Nov 23. J Cell Physiol. 2019. PMID: 30471096 Review.
Cited by
-
Actions and interactions of IGF-I and MMPs during muscle regeneration.Semin Cell Dev Biol. 2021 Nov;119:11-22. doi: 10.1016/j.semcdb.2021.04.018. Epub 2021 May 5. Semin Cell Dev Biol. 2021. PMID: 33962867 Free PMC article. Review.
-
Emerging role of vitamin D3 in alleviating intestinal structure injury caused by Aeromonas hydrophila in grass carp (Ctenopharyngodon idella).Anim Nutr. 2023 Dec 20;16:202-217. doi: 10.1016/j.aninu.2023.07.010. eCollection 2024 Mar. Anim Nutr. 2023. PMID: 38362511 Free PMC article.
-
The myokine Fibcd1 is an endogenous determinant of myofiber size and mitigates cancer-induced myofiber atrophy.Nat Commun. 2022 May 2;13(1):2370. doi: 10.1038/s41467-022-30120-1. Nat Commun. 2022. PMID: 35501350 Free PMC article.
-
Sox11 is enriched in myogenic progenitors but dispensable for development and regeneration of the skeletal muscle.Skelet Muscle. 2023 Sep 13;13(1):15. doi: 10.1186/s13395-023-00324-0. Skelet Muscle. 2023. PMID: 37705115 Free PMC article.
-
ATF3 induction prevents precocious activation of skeletal muscle stem cell by regulating H2B expression.Nat Commun. 2023 Aug 17;14(1):4978. doi: 10.1038/s41467-023-40465-w. Nat Commun. 2023. PMID: 37591871 Free PMC article.
References
-
- Beerman I, Bock C, Garrison BS, Smith ZD, Gu H, Meissner A, Rossi DJ. Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell stem cell. 2013;12:413–425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

