Phagosomal Neutralization by the Fungal Pathogen Candida albicans Induces Macrophage Pyroptosis
- PMID: 27872238
- PMCID: PMC5278172
- DOI: 10.1128/IAI.00832-16
Phagosomal Neutralization by the Fungal Pathogen Candida albicans Induces Macrophage Pyroptosis
Abstract
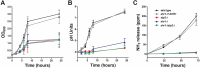
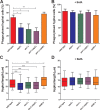
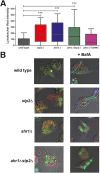
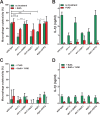
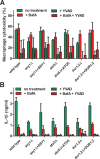
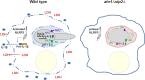
The interaction of Candida albicans with the innate immune system is the key determinant of the pathogen/commensal balance and has selected for adaptations that facilitate the utilization of nutrients commonly found within the host, including proteins and amino acids; many of the catabolic pathways needed to assimilate these compounds are required for persistence in the host. We have shown that C. albicans co-opts amino acid catabolism to generate and excrete ammonia, which raises the extracellular pH, both in vitro and in vivo and induces hyphal morphogenesis. Mutants defective in the uptake or utilization of amino acids, such as those lacking STP2, a transcription factor that regulates the expression of amino acid permeases, are impaired in multiple aspects of fungus-macrophage interactions resulting from an inability to neutralize the phagosome. Here we identified a novel role in amino acid utilization for Ahr1p, a transcription factor previously implicated in regulation of adherence and hyphal morphogenesis. Mutants lacking AHR1 were defective in growth, alkalinization, and ammonia release on amino acid-rich media, similar to stp2Δ and ahr1Δ stp2Δ cells, and occupied more acidic phagosomes. Notably, ahr1Δ and stp2Δ strains did not induce pyroptosis, as measured by caspase-1-dependent interleukin-1β release, though this phenotype could be suppressed by pharmacological neutralization of the phagosome. Altogether, we show that C. albicans-driven neutralization of the phagosome promotes hyphal morphogenesis, sufficient for induction of caspase-1-mediated macrophage lysis.
Keywords: Ahr1; Candida albicans; alkalinization; macrophages; pyroptosis.
Copyright © 2017 American Society for Microbiology.
Figures
Similar articles
-
Modulation of phagosomal pH by Candida albicans promotes hyphal morphogenesis and requires Stp2p, a regulator of amino acid transport.PLoS Pathog. 2014 Mar 13;10(3):e1003995. doi: 10.1371/journal.ppat.1003995. eCollection 2014 Mar. PLoS Pathog. 2014. PMID: 24626429 Free PMC article.
-
Candida albicans morphogenesis is not required for macrophage interleukin 1β production.mBio. 2012 Dec 26;4(1):e00433-12. doi: 10.1128/mBio.00433-12. mBio. 2012. PMID: 23269828 Free PMC article.
-
Glutamate dehydrogenase (Gdh2)-dependent alkalization is dispensable for escape from macrophages and virulence of Candida albicans.PLoS Pathog. 2020 Sep 16;16(9):e1008328. doi: 10.1371/journal.ppat.1008328. eCollection 2020 Sep. PLoS Pathog. 2020. PMID: 32936835 Free PMC article.
-
Fungal pathogen recognition by the NLRP3 inflammasome.Virulence. 2010 Jul-Aug;1(4):276-80. doi: 10.4161/viru.1.4.11482. Virulence. 2010. PMID: 21178453 Review.
-
Messenger RNA transport in the opportunistic fungal pathogen Candida albicans.Curr Genet. 2017 Dec;63(6):989-995. doi: 10.1007/s00294-017-0707-6. Epub 2017 May 16. Curr Genet. 2017. PMID: 28512683 Free PMC article. Review.
Cited by
-
Adaptation of Candida albicans to specific host environments by gain-of-function mutations in transcription factors.PLoS Pathog. 2024 Nov 4;20(11):e1012643. doi: 10.1371/journal.ppat.1012643. eCollection 2024 Nov. PLoS Pathog. 2024. PMID: 39495716 Free PMC article. Review.
-
Non-albicans Candida Species: Immune Response, Evasion Mechanisms, and New Plant-Derived Alternative Therapies.J Fungi (Basel). 2022 Dec 21;9(1):11. doi: 10.3390/jof9010011. J Fungi (Basel). 2022. PMID: 36675832 Free PMC article. Review.
-
Cytosolic Recognition of Microbes and Pathogens: Inflammasomes in Action.Microbiol Mol Biol Rev. 2018 Sep 12;82(4):e00015-18. doi: 10.1128/MMBR.00015-18. Print 2018 Dec. Microbiol Mol Biol Rev. 2018. PMID: 30209070 Free PMC article. Review.
-
A Genome-Wide Screen of Deletion Mutants in the Filamentous Saccharomyces cerevisiae Background Identifies Ergosterol as a Direct Trigger of Macrophage Pyroptosis.mBio. 2018 Jul 31;9(4):e01204-18. doi: 10.1128/mBio.01204-18. mBio. 2018. PMID: 30065091 Free PMC article.
-
Multiple Alternative Carbon Pathways Combine To Promote Candida albicans Stress Resistance, Immune Interactions, and Virulence.mBio. 2020 Jan 14;11(1):e03070-19. doi: 10.1128/mBio.03070-19. mBio. 2020. PMID: 31937647 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

