Cancer cell CCL5 mediates bone marrow independent angiogenesis in breast cancer
- PMID: 27863423
- PMCID: PMC5356747
- DOI: 10.18632/oncotarget.13387
Cancer cell CCL5 mediates bone marrow independent angiogenesis in breast cancer
Abstract
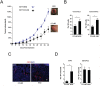
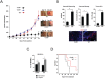
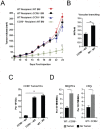
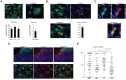
It has recently been suggested that the chemokine receptor (CCR5) is required for bone marrow (BM) derived endothelial progenitor cell (EPC) mediated angiogenesis. Here we show that suppression of either cancer cell produced CCL5, or host CCR5 leads to distinctive vascular and tumor growth defects in breast cancer. Surprisingly, CCR5 restoration in the BM alone was not sufficient to rescue the wild type phenotype, suggesting that impaired tumor growth associated with inhibiting CCL5/CCR5 is not due to defects in EPC biology. Instead, to promote angiogenesis cancer cell CCL5 may signal directly to endothelium in the tumor-stroma. In support of this hypothesis, we have also shown: (i) that endothelial cell CCR5 levels increases in response to tumor-conditioned media; (ii) that the amount of CCR5+ tumor vasculature correlates with invasive grade; and (iii) that inhibition of CCL5/CCR5 signaling impairs endothelial cell migration, associated with a decrease in activation of mTOR/AKT pathway members. Finally, we show that treatment with CCR5 antagonist results in less vasculature, impaired tumor growth, reduced metastases and improved survival. Taken as a whole, this work demonstrates that directly inhibiting CCR5 expressing vasculature constitutes a novel strategy for inhibiting angiogenesis and blocking metastatic progression in breast cancer.
Keywords: CCL5; CCR5; angiogenesis; breast cancer; shRNAi.
Conflict of interest statement
The authors whose names are listed immediately above certify that they have NO affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
Figures
Similar articles
-
CCL5/CCR5 axis induces vascular endothelial growth factor-mediated tumor angiogenesis in human osteosarcoma microenvironment.Carcinogenesis. 2015 Jan;36(1):104-14. doi: 10.1093/carcin/bgu218. Epub 2014 Oct 20. Carcinogenesis. 2015. PMID: 25330803
-
Endothelial cells promote triple-negative breast cancer cell metastasis via PAI-1 and CCL5 signaling.FASEB J. 2018 Jan;32(1):276-288. doi: 10.1096/fj.201700237RR. Epub 2017 Sep 12. FASEB J. 2018. PMID: 28899878
-
The potential to target CCL5/CCR5 in breast cancer.Expert Opin Ther Targets. 2014 Nov;18(11):1265-75. doi: 10.1517/14728222.2014.949238. Epub 2014 Sep 26. Expert Opin Ther Targets. 2014. PMID: 25256399 Review.
-
Ovarian cancer stem-like cells differentiate into endothelial cells and participate in tumor angiogenesis through autocrine CCL5 signaling.Cancer Lett. 2016 Jun 28;376(1):137-47. doi: 10.1016/j.canlet.2016.03.034. Epub 2016 Mar 23. Cancer Lett. 2016. PMID: 27033454
-
Inhibition of the CCL5/CCR5 Axis against the Progression of Gastric Cancer.Int J Mol Sci. 2018 May 16;19(5):1477. doi: 10.3390/ijms19051477. Int J Mol Sci. 2018. PMID: 29772686 Free PMC article. Review.
Cited by
-
Cancer-Associated Adipocytes and Breast Cancer: Intertwining in the Tumor Microenvironment and Challenges for Cancer Therapy.Cancers (Basel). 2023 Jan 24;15(3):726. doi: 10.3390/cancers15030726. Cancers (Basel). 2023. PMID: 36765683 Free PMC article. Review.
-
G Protein-Coupled Receptors at the Crossroad between Physiologic and Pathologic Angiogenesis: Old Paradigms and Emerging Concepts.Int J Mol Sci. 2017 Dec 14;18(12):2713. doi: 10.3390/ijms18122713. Int J Mol Sci. 2017. PMID: 29240722 Free PMC article. Review.
-
The CCL5/CCR5 Axis in Cancer Progression.Cancers (Basel). 2020 Jul 2;12(7):1765. doi: 10.3390/cancers12071765. Cancers (Basel). 2020. PMID: 32630699 Free PMC article. Review.
-
Exosomal MicroRNAs as Mediators of Cellular Interactions Between Cancer Cells and Macrophages.Front Immunol. 2020 Jun 11;11:1167. doi: 10.3389/fimmu.2020.01167. eCollection 2020. Front Immunol. 2020. PMID: 32595638 Free PMC article. Review.
-
CC chemokines are differentially expressed in Breast Cancer and are associated with disparity in overall survival.Sci Rep. 2019 Mar 8;9(1):4014. doi: 10.1038/s41598-019-40514-9. Sci Rep. 2019. PMID: 30850664 Free PMC article.
References
-
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–8. - PubMed
-
- Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. EPCs control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–8. - PubMed
-
- Mellick AS, Plummer PN, Nolan DJ, Gao D, Bambino K, Hahn M, Catena R, Turner V, McDonnell K, Benezra R, Brink R, Swarbrick A, Mittal V. Using the transcription factor inhibitor of DNA binding 1 to selectively target endothelial progenitor cells offers novel strategies to inhibit tumor angiogenesis and growth. Cancer Res. 2010;70:7273–82. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

