Inflammatory Properties and Adjuvant Potential of Synthetic Glycolipids Homologous to Mycolate Esters of the Cell Wall of Mycobacterium tuberculosis
- PMID: 27855374
- PMCID: PMC6738904
- DOI: 10.1159/000450955
Inflammatory Properties and Adjuvant Potential of Synthetic Glycolipids Homologous to Mycolate Esters of the Cell Wall of Mycobacterium tuberculosis
Abstract
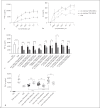
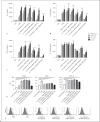
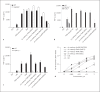
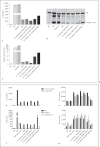
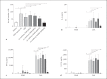
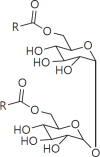
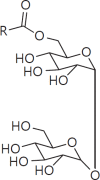
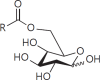
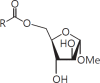
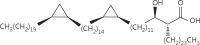
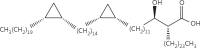
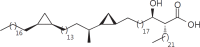
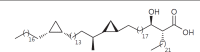
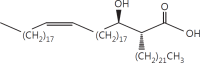
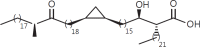
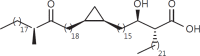
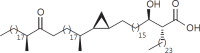
The cell wall of mycobacteria is characterised by glycolipids composed of different classes of mycolic acids (MAs; alpha-, keto-, and methoxy-) and sugars (trehalose, glucose, and arabinose). Studies using mutant Mtb strains have shown that the structure of MAs influences the inflammatory potential of these glycolipids. As mutant Mtb strains possess a complex mixture of glycolipids, we analysed the inflammatory potential of single classes of mycolate esters of the Mtb cell wall using 38 different synthetic analogues. Our results show that synthetic trehalose dimycolate (TDM) and trehalose, glucose, and arabinose monomycolates (TMM, GMM, and AraMM) activate bone marrow-derived dendritic cells in terms of the production of pro-inflammatory cytokines (IL-6 and TNF-α) and reactive oxygen species, upregulation of costimulatory molecules, and activation of NLRP3 inflammasome by a mechanism dependent on Mincle. These findings demonstrate that Mincle receptor can also recognise pentose esters and seem to contradict the hypothesis that production of GMM is an escape mechanism used by pathogenic mycobacteria to avoid recognition by the innate immune system. Finally, our experiments indicate that TMM and GMM, as well as TDM, can promote Th1 and Th17 responses in mice in an OVA immunisation model, and that further analysis of their potential as novel adjuvants for subunit vaccines is warranted.
© 2016 S. Karger AG, Basel.
Figures


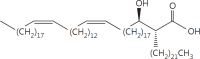
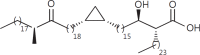
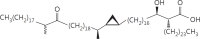
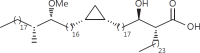
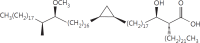


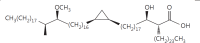
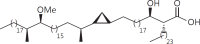

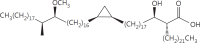
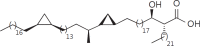
Similar articles
-
The mycobacterial cord factor adjuvant analogue trehalose-6,6'-dibehenate (TDB) activates the Nlrp3 inflammasome.Immunobiology. 2013 Apr;218(4):664-73. doi: 10.1016/j.imbio.2012.07.029. Epub 2012 Aug 7. Immunobiology. 2013. PMID: 22921586
-
C-Type Lectin Receptor MCL Facilitates Mincle Expression and Signaling through Complex Formation.J Immunol. 2015 Jun 1;194(11):5366-74. doi: 10.4049/jimmunol.1402429. Epub 2015 Apr 17. J Immunol. 2015. PMID: 25888641
-
Trehalose diester glycolipids are superior to the monoesters in binding to Mincle, activation of macrophages in vitro and adjuvant activity in vivo.Innate Immun. 2016 Aug;22(6):405-18. doi: 10.1177/1753425916651132. Epub 2016 Jun 1. Innate Immun. 2016. PMID: 27252171 Free PMC article.
-
Recognition of Mycobacterial Lipids by Immune Receptors.Trends Immunol. 2017 Jan;38(1):66-76. doi: 10.1016/j.it.2016.10.009. Epub 2016 Nov 23. Trends Immunol. 2017. PMID: 27889398 Review.
-
[The 72nd Annual Meeting Education Lecture. Cord factor].Kekkaku. 1998 Jan;73(1):37-42. Kekkaku. 1998. PMID: 9494342 Review. Japanese.
Cited by
-
Versatile approach towards fully desymmetrized trehalose with a novel set of orthogonal protecting groups.Front Chem. 2024 Jan 11;11:1332837. doi: 10.3389/fchem.2023.1332837. eCollection 2023. Front Chem. 2024. PMID: 38274896 Free PMC article.
-
Persistence of Mycobacterium tuberculosis in response to infection burden and host-induced stressors.Front Cell Infect Microbiol. 2022 Dec 2;12:981827. doi: 10.3389/fcimb.2022.981827. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36530432 Free PMC article.
-
Identification and Biological Activity of Synthetic Macrophage Inducible C-Type Lectin Ligands.Front Immunol. 2018 Jan 17;8:1940. doi: 10.3389/fimmu.2017.01940. eCollection 2017. Front Immunol. 2018. PMID: 29387054 Free PMC article. Review.
-
The C-Type Lectin Mincle: Clues for a Role in Crohn's Disease Adjuvant Reaction.Front Immunol. 2017 Oct 23;8:1304. doi: 10.3389/fimmu.2017.01304. eCollection 2017. Front Immunol. 2017. PMID: 29109721 Free PMC article. No abstract available.
-
Elevated serum antibody responses to synthetic mycobacterial lipid antigens among UK farmers: an indication of exposure to environmental mycobacteria?RSC Med Chem. 2020 Nov 26;12(2):213-221. doi: 10.1039/d0md00325e. eCollection 2021 Mar 4. RSC Med Chem. 2020. PMID: 34046610 Free PMC article.
References
-
- Minnikin DE, Kremer L, Dover LG, Besra GS. The methyl-branched fortifications of Mycobacterium tuberculosis. Chem Biol. 2002;9:545–553. - PubMed
-
- Verschoor JA, Baird MS, Grooten J. Towards understanding the functional diversity of cell wall mycolic acids of Mycobacterium tuberculosis. Prog Lipid Res. 2012;51:325–339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

