Macrophage-derived IL-1β enhances monosodium urate crystal-triggered NET formation
- PMID: 27853847
- PMCID: PMC5296223
- DOI: 10.1007/s00011-016-1008-0
Macrophage-derived IL-1β enhances monosodium urate crystal-triggered NET formation
Abstract
Objective and design: Arthritic gout is caused by joint inflammation triggered by the damaging effects of monosodium uric acid (MSU) crystal accumulation in the synovial space. Neutrophils play a major role in mediating joint inflammation in gout. Along with neutrophils, other immune cells, such as macrophages, are present in inflamed joints and contribute to gout pathogenesis. Neutrophils form neutrophil extracellular traps (NETs) in response to MSU crystals. In the presence of MSU crystals, macrophages release IL-1β, a cytokine crucial to initiate gout pathogenesis and neutrophil recruitment. Our research investigated interactions between human macrophages and neutrophils in an in vitro model system and asked how macrophages affect NET formation stimulated by MSU crystals.
Materials or subjects: Human neutrophils and PBMCs were isolated from peripheral blood of healthy volunteers. PBMCs were differentiated into macrophages in vitro using human M-CSF.
Treatment: Human neutrophils were pretreated with macrophage-conditioned media, neutrophil-conditioned media, recombinant human IL-1β or anakinra prior to stimulation by MSU crystals.
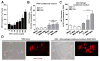
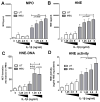
Method: Interaction of neutrophils with MSU crystals was evaluated by live imaging using confocal microscopy. The presence of myeloperoxidase (MPO) and neutrophil elastase (NE) was measured by ELISA. NET formation was quantitated by Sytox Orange-based extracellular DNA release assay and NE-DNA ELISA. AggNET formation was assessed by macroscopic evaluation.
Results: We found that crystal- and cell-free supernatants of macrophages stimulated with MSU crystals promote MSU crystal-stimulated NET formation in human neutrophils. This observation was confirmed by additional assays measuring the release of MPO, NE, and the enzymatic activity of NE. MSU crystal-induced NET formation remained unchanged when neutrophil supernatants were tested. IL-1β is a crucial cytokine orchestrating the onset of inflammation in gout and is known to be released in large amounts from macrophages following MSU crystal stimulation. We found that recombinant IL-1β strongly promoted MSU crystal-induced NET formation in human neutrophils. Interestingly, IL-1β alone did not induce any NET release. We also found that clinical grade anakinra, an IL-1 receptor blocker, strongly reduced the NETosis-enhancing effect of macrophage supernatants indicating that IL-1β is mainly responsible for this effect.
Conclusions: Macrophage-derived IL-1β enhances MSU crystal-induced NET release in neutrophils. We identified a new mechanism by which macrophages and IL-1β affect neutrophil functions, and could contribute to the inflammatory conditions present in gout. Our results also revealed a new anti-inflammatory mechanism of anakinra.
Keywords: Gout; Interleukin-1 beta; Macrophage; Neutrophil extracellular traps (NETs).
Conflict of interest statement
The authors have no financial conflicts of interest to report.
Figures
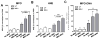
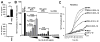

Similar articles
-
P2Y6 Receptor Antagonist MRS2578 Inhibits Neutrophil Activation and Aggregated Neutrophil Extracellular Trap Formation Induced by Gout-Associated Monosodium Urate Crystals.J Immunol. 2017 Jan 1;198(1):428-442. doi: 10.4049/jimmunol.1600766. Epub 2016 Nov 30. J Immunol. 2017. PMID: 27903742
-
NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B(4) in a murine model of gout.Arthritis Rheum. 2012 Feb;64(2):474-84. doi: 10.1002/art.33355. Arthritis Rheum. 2012. PMID: 21952942
-
Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout.Arthritis Rheum. 2009 Jan;60(1):281-9. doi: 10.1002/art.24185. Arthritis Rheum. 2009. PMID: 19116939
-
How neutrophil extracellular traps orchestrate the local immune response in gout.J Mol Med (Berl). 2015 Jul;93(7):727-34. doi: 10.1007/s00109-015-1295-x. Epub 2015 May 24. J Mol Med (Berl). 2015. PMID: 26002146 Review.
-
Neutrophils, IL-1β, and gout: is there a link?Semin Immunopathol. 2013 Jul;35(4):501-12. doi: 10.1007/s00281-013-0361-0. Epub 2013 Jan 24. Semin Immunopathol. 2013. PMID: 23344781 Review.
Cited by
-
Covid-19: Perspectives on Innate Immune Evasion.Front Immunol. 2020 Sep 30;11:580641. doi: 10.3389/fimmu.2020.580641. eCollection 2020. Front Immunol. 2020. PMID: 33101306 Free PMC article. Review.
-
Co-regulated ceRNA network mediated by circRNA and lncRNA in patients with gouty arthritis.BMC Med Genomics. 2024 Nov 7;17(1):264. doi: 10.1186/s12920-024-02038-8. BMC Med Genomics. 2024. PMID: 39511617 Free PMC article.
-
Anti-inflammatory Properties of Tongfeng Li'an Granules in an Acute Gouty Arthritis Rat Model.ACS Omega. 2024 Jul 31;9(32):34303-34313. doi: 10.1021/acsomega.4c00056. eCollection 2024 Aug 13. ACS Omega. 2024. PMID: 39157086 Free PMC article.
-
1-Palmitoyl-2-Linoleoyl-3-Acetyl-rac-Glycerol (PLAG) Mitigates Monosodium Urate (MSU)-Induced Acute Gouty Inflammation in BALB/c Mice.Front Immunol. 2020 Apr 24;11:710. doi: 10.3389/fimmu.2020.00710. eCollection 2020. Front Immunol. 2020. PMID: 32395118 Free PMC article.
-
Neutrophil extracellular traps in homeostasis and disease.Signal Transduct Target Ther. 2024 Sep 20;9(1):235. doi: 10.1038/s41392-024-01933-x. Signal Transduct Target Ther. 2024. PMID: 39300084 Free PMC article. Review.
References
-
- Busso N, So A. Microcrystals as DAMPs and their role in joint inflammation. Rheumatology (Oxford) 2012;51:1154–60. - PubMed
-
- Neogi T. Clinical practice. Gout N Engl J Med. 2011;364:443–52. - PubMed
-
- Mitroulis I, Kambas K, Ritis K. Neutrophils, IL-1beta, and gout: is there a link? Semin Immunopathol. 2013;35:501–12. - PubMed
-
- Pope RM, Tschopp J. The role of interleukin-1 and the inflammasome in gout: implications for therapy. Arthritis Rheum. 2007;56:3183–8. - PubMed
-
- Schauer C, Janko C, Munoz LE, Zhao Y, Kienhofer D, Frey B, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20:511–7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

