Safety and immunogenicity of the PRAME cancer immunotherapeutic in metastatic melanoma: results of a phase I dose escalation study
- PMID: 27843625
- PMCID: PMC5070281
- DOI: 10.1136/esmoopen-2016-000068
Safety and immunogenicity of the PRAME cancer immunotherapeutic in metastatic melanoma: results of a phase I dose escalation study
Abstract
Purpose: The PRAME tumour antigen is expressed in several tumour types but in few normal adult tissues. A dose-escalation phase I/II study (NCT01149343) assessed the safety, immunogenicity and clinical activity of the PRAME immunotherapeutic (recombinant PRAME protein (recPRAME) with the AS15 immunostimulant) in patients with advanced melanoma. Here, we report the phase I dose-escalation study segment.
Patients and methods: Patients with stage IV PRAME-positive melanoma were enrolled to 3 consecutive cohorts to receive up to 24 intramuscular injections of the PRAME immunotherapeutic. The RecPRAME dose was 20, 100 or 500 µg in cohorts 1, 2 and 3, respectively, with a fixed dose of AS15. Adverse events (AEs), including predefined dose-limiting toxicity (DLT) and the anti-PRAME humoral response (ELISA), were coprimary end points. Cellular immune responses were evaluated using in vitro assays.
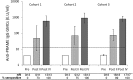
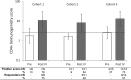
Results: 66 patients were treated (20, 24 and 22 in the respective cohorts). AEs considered by the investigator to be causally related were mostly grade 1 or 2 injection site symptoms, fatigue, chills, fever and headache. Two DLTs (grade 3 brain oedema and proteinuria) were recorded in two patients in two cohorts (cohorts 2 and 3). All patients had detectable anti-PRAME antibodies after four immunisations. Percentages of patients with predefined PRAME-specific-CD4+T-cell responses after four immunisations were similar in each cohort. No CD8+ T-cell responses were detected.
Conclusions: The PRAME immunotherapeutic had an acceptable safety profile and induced similar anti-PRAME-specific humoral and cellular immune responses in all cohorts. As per protocol, the phase II study segment was initiated to further evaluate the 500 µg PRAME immunotherapeutic dose.
Trial registration number: NCT01149343, Results.
Keywords: PRAME antigen; cancer immunotherapy; immunogenicity; metastatic melanoma; safety.
Conflict of interest statement
Employment: MG (GSK group of companies); NV (GSK group of companies); BS (GSK group of companies); SJ (GSK group of companies); PMDeSA (GSK group of companies); FFL (GSK group of companies); JL (GSK group of companies); VGB (GSK group of companies). Stock Ownership: SJ (GSK group of companies), FFL (GSK group of companies); JL (GSK group of companies); VGB (GSK group of companies); PMDeSA (GSK group of companies). Honoraria: BD (Roche, GSK, BMS), DS (GSK, Roche, BMS, Amgen, Novartis, Merck/MSD), JJG (GSK, BMS, Roche, Amgen, MSD, Novartis, Meda), AT (BMS, GSK, Amgen, Roche), AH (Amgen, BMS, Celgene, Eisai, GSK, MedImmune, MelaSciences, Merck Serono, MSD/Merck, Novartis, Oncosec, Roche Pharma), RG (GSK, Roche, BMS, MSD, Novartis, Pfizer, Janssen, Amgen, Merck Serono, Boehringer, Almirall Hermal), JU (Roche, GSK, BMS), PA (BMS, Roche/Genentech, GSK, Ventana), RR (BMS, GSK), ES (Novartis, BMS, DermaPharm), TL (GSK, BMS, Merck, Roche). Consultant or Advisory Role: BD (Roche, BMS, GSK), DS (GSK, Roche, BMS, Amgen, Novartis, Merck/MSD), JJG (GSK, BMS, Roche, Amgen, MSD, Novartis, Meda), AT (BMS, GSK, Amgen, Roche), AH (Amgen, BMS, Celgene, Eisai, GSK, MedImmune, MelaSciences, Merck Serono, MSD/Merck, Novartis, Oncosec, Roche Pharma), RG (GSK, BMS, Roche, Novartis, Almirall Hermal, MSD, Amgen), JU (Roche, GSK), PA (BMS, Roche/Genentech, MSD, GSK, Ventana, Novartis, Amgen), PQ (GSK, Roche, Bristol, MSD), CL (Roche, BMS, MSD), ES (BMS). Speakers’ Bureau: DS (GSK; Roche, BMS, Amgen, Novartis, Merck/MSD), JJG (GSK), RG (GSK), JU (Roche), CL (Roche, BMS, MSD, Leo), TL (GSK, Merck, Roche), LD (GSK, BMS, Roche, MSD). Research funding: BD (Roche, GSK), DS (Merck), JJG (Roche), AH (trial grants from Amgen, BMS, Celgene, Eisai, GSK, MelaSciences, Merck Serono, MSD/Merck, Novartis, Oncosec, Roche Pharma), RG (Roche, Novartis, Pfizer, Johnson & Johnson), PA (BMS, Roche/Genentech, Ventana). Patents, royalties, other intellectual property: ES (Royalties from Ludwig Institute for Cancer Research for contribution to a patent on human tumor antigen). Travel, accommodations, expenses: BD (Roche, BMS), DS (GSK, Roche, BMS, Amgen, Novartis, Merck/MSD), AT (Oncovision), RG (Roche, BMS), JU (Roche, GSK), PQ (Roche, GSK, MSD), CL (Roche, BMS, Leo), TL (Roche). EL, LR, AS declare that they have no conflict of interest.
Figures
Similar articles
-
Safety and Immunogenicity of the PRAME Cancer Immunotherapeutic in Patients with Resected Non-Small Cell Lung Cancer: A Phase I Dose Escalation Study.J Thorac Oncol. 2016 Dec;11(12):2208-2217. doi: 10.1016/j.jtho.2016.08.120. Epub 2016 Aug 17. J Thorac Oncol. 2016. PMID: 27544054 Clinical Trial.
-
A Comprehensive Preclinical Model Evaluating the Recombinant PRAME Antigen Combined With the AS15 Immunostimulant to Fight Against PRAME-expressing Tumors.J Immunother. 2015 Oct;38(8):311-20. doi: 10.1097/CJI.0000000000000095. J Immunother. 2015. PMID: 26325375 Free PMC article.
-
A non-randomized dose-escalation Phase I trial of a protein-based immunotherapeutic for the treatment of breast cancer patients with HER2-overexpressing tumors.Breast Cancer Res Treat. 2016 Apr;156(2):319-30. doi: 10.1007/s10549-016-3751-x. Epub 2016 Mar 18. Breast Cancer Res Treat. 2016. PMID: 26993131 Clinical Trial.
-
Cancer Testis Antigens and Immunotherapy: Where Do We Stand in the Targeting of PRAME?Cancers (Basel). 2019 Jul 15;11(7):984. doi: 10.3390/cancers11070984. Cancers (Basel). 2019. PMID: 31311081 Free PMC article. Review.
-
The role of the cancer testis antigen PRAME in tumorigenesis and immunotherapy in human cancer.Cell Prolif. 2020 Mar;53(3):e12770. doi: 10.1111/cpr.12770. Epub 2020 Feb 5. Cell Prolif. 2020. PMID: 32022332 Free PMC article. Review.
Cited by
-
The Utilization of PRAME in the Diagnosis, Prognosis, and Treatment of Melanoma.Cells. 2024 Oct 20;13(20):1740. doi: 10.3390/cells13201740. Cells. 2024. PMID: 39451258 Free PMC article. Review.
-
PRAME Staining in Sinonasal Mucosal Melanoma: A Single-Center Experience.Head Neck Pathol. 2023 Jun;17(2):401-408. doi: 10.1007/s12105-022-01515-9. Epub 2022 Dec 31. Head Neck Pathol. 2023. PMID: 36586078 Free PMC article.
-
Immunohistochemistry for PRAME in Dermatopathology.Am J Dermatopathol. 2023 Nov 1;45(11):733-747. doi: 10.1097/DAD.0000000000002440. Am J Dermatopathol. 2023. PMID: 37856737 Free PMC article.
-
PRAME Is an Effective Tool for the Diagnosis of Nevus-Associated Cutaneous Melanoma.Cancers (Basel). 2024 Jan 9;16(2):278. doi: 10.3390/cancers16020278. Cancers (Basel). 2024. PMID: 38254769 Free PMC article.
-
PRAME Expression in Melanocytic Tumors.Am J Surg Pathol. 2018 Nov;42(11):1456-1465. doi: 10.1097/PAS.0000000000001134. Am J Surg Pathol. 2018. PMID: 30045064 Free PMC article.
References
-
- Ikeda H, Lethé B, Lehmann F et al. . Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity 1997;6:199–208. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

