Paraventricular Hypothalamic Mechanisms of Chronic Stress Adaptation
- PMID: 27843437
- PMCID: PMC5086584
- DOI: 10.3389/fendo.2016.00137
Paraventricular Hypothalamic Mechanisms of Chronic Stress Adaptation
Abstract
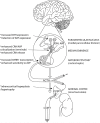
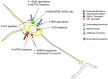
The hypothalamic paraventricular nucleus (PVN) is the primary driver of hypothalamo-pituitary-adrenocortical (HPA) responses. At least part of the role of the PVN is managing the demands of chronic stress exposure. With repeated exposure to stress, hypophysiotrophic corticotropin-releasing hormone (CRH) neurons of the PVN display a remarkable cellular, synaptic, and connectional plasticity that serves to maximize the ability of the HPA axis to maintain response vigor and flexibility. At the cellular level, chronic stress enhances the production of CRH and its co-secretagogue arginine vasopressin and rearranges neurotransmitter receptor expression so as to maximize cellular excitability. There is also evidence to suggest that efficacy of local glucocorticoid feedback is reduced following chronic stress. At the level of the synapse, chronic stress enhances cellular excitability and reduces inhibitory tone. Finally, chronic stress causes a structural enhancement of excitatory innervation, increasing the density of glutamate and noradrenergic/adrenergic terminals on CRH neuronal cell somata and dendrites. Together, these neuroplastic changes favor the ability of the HPA axis to retain responsiveness even under conditions of considerable adversity. Thus, chronic stress appears able to drive PVN neurons via a number of convergent mechanisms, processes that may play a major role in HPA axis dysfunction seen in variety of stress-linked disease states.
Keywords: corticotropin-releasing hormone; glucocorticoids; hypothalamo–pituitary–adrenal axis; neuroendocrine system; vasopressin.
Figures
Similar articles
-
The stress system in the human brain in depression and neurodegeneration.Ageing Res Rev. 2005 May;4(2):141-94. doi: 10.1016/j.arr.2005.03.003. Ageing Res Rev. 2005. PMID: 15996533 Review.
-
Chronic stress dampens excitatory synaptic gain in the paraventricular nucleus of the hypothalamus.J Physiol. 2018 Sep;596(17):4157-4172. doi: 10.1113/JP275669. Epub 2018 Jul 22. J Physiol. 2018. PMID: 29901836 Free PMC article.
-
Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels.Endocrinology. 1995 Aug;136(8):3299-309. doi: 10.1210/endo.136.8.7628364. Endocrinology. 1995. PMID: 7628364
-
Regulation of basal corticotropin-releasing hormone and arginine vasopressin messenger ribonucleic acid expression in the paraventricular nucleus: effects of selective hypothalamic deafferentations.Endocrinology. 1990 Nov;127(5):2408-17. doi: 10.1210/endo-127-5-2408. Endocrinology. 1990. PMID: 2171915
-
Stress-induced norepinephrine release in the hypothalamic paraventricular nucleus and pituitary-adrenocortical and sympathoadrenal activity: in vivo microdialysis studies.Front Neuroendocrinol. 1995 Apr;16(2):89-150. doi: 10.1006/frne.1995.1004. Front Neuroendocrinol. 1995. PMID: 7621982 Review.
Cited by
-
The Interaction of Vasopressin with Hormones of the Hypothalamo-Pituitary-Adrenal Axis: The Significance for Therapeutic Strategies in Cardiovascular and Metabolic Diseases.Int J Mol Sci. 2024 Jul 5;25(13):7394. doi: 10.3390/ijms25137394. Int J Mol Sci. 2024. PMID: 39000501 Free PMC article. Review.
-
The Neurobiological Links between Stress and Traumatic Brain Injury: A Review of Research to Date.Int J Mol Sci. 2022 Aug 23;23(17):9519. doi: 10.3390/ijms23179519. Int J Mol Sci. 2022. PMID: 36076917 Free PMC article. Review.
-
Clinical characteristics of patients with unexplainable hypothalamic disorder diagnosed by the corticotropin-releasing hormone challenge test: a retrospective study.BMC Endocr Disord. 2022 Dec 9;22(1):312. doi: 10.1186/s12902-022-01237-7. BMC Endocr Disord. 2022. PMID: 36494805 Free PMC article.
-
The Effect of Craniosacral Therapy on Blood Levels of Stress Hormones in Male Firefighter Cadets: A Randomized Clinical Trial.Behav Sci (Basel). 2023 Nov 8;13(11):914. doi: 10.3390/bs13110914. Behav Sci (Basel). 2023. PMID: 37998661 Free PMC article.
-
Stress Adaptation Upregulates Oxytocin within Hypothalamo-Vagal Neurocircuits.Neuroscience. 2018 Oct 15;390:198-205. doi: 10.1016/j.neuroscience.2018.08.021. Epub 2018 Aug 31. Neuroscience. 2018. PMID: 30176320 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

