The common mouse protozoa Tritrichomonas muris alters mucosal T cell homeostasis and colitis susceptibility
- PMID: 27836928
- PMCID: PMC5154950
- DOI: 10.1084/jem.20161776
The common mouse protozoa Tritrichomonas muris alters mucosal T cell homeostasis and colitis susceptibility
Abstract
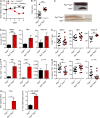
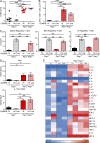
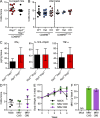
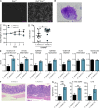
The mammalian gastrointestinal tract hosts a diverse community of microbes including bacteria, fungi, protozoa, helminths, and viruses. Through coevolution, mammals and these microbes have developed a symbiosis that is sustained through the host's continuous sensing of microbial factors and the generation of a tolerant or pro-inflammatory response. While analyzing T cell-driven colitis in nonlittermate mouse strains, we serendipitously identified that a nongenetic transmissible factor dramatically increased disease susceptibility. We identified the protozoan Tritrichomonas muris as the disease-exacerbating element. Furthermore, experimental colonization with T. muris induced an elevated Th1 response in the cecum of naive wild-type mice and accelerated colitis in Rag1-/- mice after T cell transfer. Overall, we describe a novel cross-kingdom interaction within the murine gut that alters immune cell homeostasis and disease susceptibility. This example of unpredicted microbial priming of the immune response highlights the importance of studying trans-kingdom interactions and serves as a stark reminder of the importance of using littermate controls in all mouse research.
© 2016 Escalante et al.
Figures

Similar articles
-
Host-Protozoan Interactions Protect from Mucosal Infections through Activation of the Inflammasome.Cell. 2016 Oct 6;167(2):444-456.e14. doi: 10.1016/j.cell.2016.08.076. Cell. 2016. PMID: 27716507 Free PMC article.
-
Anti-Tumor Necrosis Factor With a Glyco-Engineered Fc-Region Has Increased Efficacy in Mice With Colitis.Gastroenterology. 2017 Nov;153(5):1351-1362.e4. doi: 10.1053/j.gastro.2017.07.021. Epub 2017 Jul 27. Gastroenterology. 2017. PMID: 28756234
-
Increased susceptibility to Trichuris muris infection and exacerbation of colitis in Mdr1a-/- mice.World J Gastroenterol. 2014 Feb 21;20(7):1797-806. doi: 10.3748/wjg.v20.i7.1797. World J Gastroenterol. 2014. PMID: 24587657 Free PMC article.
-
Immune regulation in the intestine: a balancing act between effector and regulatory T cell responses.Ann N Y Acad Sci. 2004 Dec;1029:132-41. doi: 10.1196/annals.1309.030. Ann N Y Acad Sci. 2004. PMID: 15681752 Review.
-
Type 1 and 2 T helper cell-mediated colitis.Curr Opin Gastroenterol. 2006 Nov;22(6):651-7. doi: 10.1097/01.mog.0000245545.80160.0f. Curr Opin Gastroenterol. 2006. PMID: 17053444 Review.
Cited by
-
A mouse protozoan boosts antigen-specific mucosal IgA responses in a specific lipid metabolism- and signaling-dependent manner.Nat Commun. 2024 Sep 10;15(1):7914. doi: 10.1038/s41467-024-52336-z. Nat Commun. 2024. PMID: 39256385 Free PMC article.
-
Tritrichomonas muris sensitizes the intestinal epithelium to doxorubicin-induced apoptosis.bioRxiv [Preprint]. 2024 Aug 9:2024.08.08.607206. doi: 10.1101/2024.08.08.607206. bioRxiv. 2024. PMID: 39149272 Free PMC article. Preprint.
-
Giardia intestinalis reshapes mucosal immunity toward a Type 2 response that attenuates inflammatory bowel-like diseases.bioRxiv [Preprint]. 2024 Mar 6:2024.03.02.583119. doi: 10.1101/2024.03.02.583119. bioRxiv. 2024. PMID: 38903060 Free PMC article. Preprint.
-
Gut protozoa of wild rodents - a meta-analysis.Parasitology. 2024 May;151(6):594-605. doi: 10.1017/S0031182024000556. Epub 2024 May 8. Parasitology. 2024. PMID: 38714350 Free PMC article.
-
A commensal protozoan attenuates Clostridioides difficile pathogenesis in mice via arginine-ornithine metabolism and host intestinal immune response.Nat Commun. 2024 Apr 2;15(1):2842. doi: 10.1038/s41467-024-47075-0. Nat Commun. 2024. PMID: 38565558 Free PMC article.
References
-
- Baker D.G. 2008. Parasites of rats and mice. In Flynn’s Parasites of Laboratory Animals. Second edition. D.G. Baker, editor. Blackwell Publishing Ltd., Ames, IA. 303–397.
-
- Cadwell K., Patel K.K., Maloney N.S., Liu T.C., Ng A.C.Y., Storer C.E., Head R.D., Xavier R., Stappenbeck T.S., and Virgin H.W.. 2010. Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell. 141:1135–1145. 10.1016/j.cell.2010.05.009 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

