Aseptically Processed Placental Membrane Improves Healing of Diabetic Foot Ulcerations: Prospective, Randomized Clinical Trial
- PMID: 27826487
- PMCID: PMC5096542
- DOI: 10.1097/GOX.0000000000001095
Aseptically Processed Placental Membrane Improves Healing of Diabetic Foot Ulcerations: Prospective, Randomized Clinical Trial
Abstract
Background: Allogeneic grafts derived from amnion/chorion are known to be efficacious in healing chronic diabetic foot ulcerations (DFUs). The goal of this study was to compare aseptically processed dehydrated human amnion and chorion allograft (dHACA) versus standard of care (SOC) in facilitating wound closure in nonhealing DFUs.
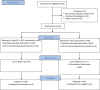
Methods: Patients with DFUs treated with SOC (off-loading, appropriate debridement, and moist wound care) after a 2-week screening period were randomized to either SOC or wound-size-specific dHACA (AmnioBand, Musculoskeletal Transplant Foundation, Edison, N.J.) applied weekly for up to 12 weeks plus SOC. Primary endpoint was the percentage of wounds healed at 6 weeks between groups.
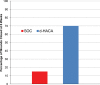
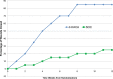
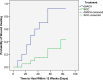
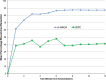
Results: At 6 weeks, 70% (14/20) of the dHACA-treated DFUs healed compared with 15% (3/20) treated with SOC alone. Furthermore, at 12 weeks, 85% (17/20) of the DFUs in the dHACA group healed compared with 25% (5/20) in the SOC group, with a corresponding mean time to heal of 36 and 70 days, respectively. At 12 weeks, the mean number of grafts used per healed wound for the dHACA group was 3.8 (median 3.0), and mean cost of the tissue to heal a DFU was $1400. The mean wastage at 12 weeks was 40%. One adverse event and 1 serious adverse event occurred in the dHACA group; neither was graft related. Three adverse events and 1 serious adverse event occurred in the SOC group.
Conclusion: Aseptically processed dHACA heals diabetic foot wounds significantly faster than SOC at 6 and 12 weeks with minimal graft wastage.
Figures

Similar articles
-
Use of an aseptically processed, dehydrated human amnion and chorion membrane improves likelihood and rate of healing in chronic diabetic foot ulcers: A prospective, randomised, multi-centre clinical trial in 80 patients.Int Wound J. 2018 Dec;15(6):950-957. doi: 10.1111/iwj.12954. Epub 2018 Jul 17. Int Wound J. 2018. PMID: 30019528 Free PMC article. Clinical Trial.
-
Placental Membrane Provides Improved Healing Efficacy and Lower Cost Versus a Tissue-Engineered Human Skin in the Treatment of Diabetic Foot Ulcerations.Plast Reconstr Surg Glob Open. 2019 Aug 30;7(8):e2371. doi: 10.1097/GOX.0000000000002371. eCollection 2019 Aug. Plast Reconstr Surg Glob Open. 2019. PMID: 31592387 Free PMC article.
-
Dehydrated Amnion Chorion Membrane versus standard of care for diabetic foot ulcers: a randomised controlled trial.J Wound Care. 2024 Jul 1;33(Sup7):S4-S14. doi: 10.12968/jowc.2024.0139. Epub 2024 Jun 6. J Wound Care. 2024. PMID: 38973638 Clinical Trial.
-
Human amniotic membrane products for patients with diabetic foot ulcers. do they help? a systematic review and meta-analysis.J Foot Ankle Res. 2022 Sep 14;15(1):71. doi: 10.1186/s13047-022-00575-y. J Foot Ankle Res. 2022. PMID: 36104736 Free PMC article. Review.
-
Human Amnion Chorion Membrane Allografts in the Treatment of Chronic Diabetic Foot Ulcers: A Literature Review.Adv Skin Wound Care. 2021 Apr 1;34(4):1-7. doi: 10.1097/01.ASW.0000734388.08779.e8. Adv Skin Wound Care. 2021. PMID: 33739952 Review.
Cited by
-
Characterisation of dehydrated amnion chorion membranes and evaluation of fibroblast and keratinocyte responses in vitro.Int Wound J. 2019 Jun;16(3):827-840. doi: 10.1111/iwj.13103. Epub 2019 Mar 10. Int Wound J. 2019. PMID: 30854789 Free PMC article.
-
Skin Substitutes for Adults With Diabetic Foot Ulcers and Venous Leg Ulcers: A Health Technology Assessment.Ont Health Technol Assess Ser. 2021 Jun 4;21(7):1-165. eCollection 2021. Ont Health Technol Assess Ser. 2021. PMID: 34211616 Free PMC article.
-
Standardized reporting of amnion and amnion/chorion allograft data for wound care.Health Sci Rep. 2022 Aug 23;5(5):e794. doi: 10.1002/hsr2.794. eCollection 2022 Sep. Health Sci Rep. 2022. PMID: 36032519 Free PMC article.
-
Clinical Evaluation of AMNIODERM+® Wound Dressing Containing Non-Viable Human Amniotic Membrane: Retrospective-Perspective Clinical Trial.BioTech (Basel). 2024 Sep 19;13(3):36. doi: 10.3390/biotech13030036. BioTech (Basel). 2024. PMID: 39311338 Free PMC article.
-
Comparison of Skin Substitutes for Acute and Chronic Wound Management.Semin Plast Surg. 2021 Aug;35(3):171-180. doi: 10.1055/s-0041-1731463. Epub 2021 Sep 10. Semin Plast Surg. 2021. PMID: 34526865 Free PMC article. Review.
References
-
- Lipsky BA, Berendt AR, Cornia PB, et al. Infectious Diseases Society of America. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132–e173. - PubMed
-
- Snyder RJ, Frykberg RG, Rogers LC, et al. The management of diabetic foot ulcers through optimal off-loading: building consensus guidelines and practical recommendations to improve outcomes. J Am Podiatr Med Assoc. 2014;104:555–567. - PubMed
-
- Hingorani A, LaMuraglia GM, Henke P, et al. The management of diabetic foot: a clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg. 2016;63(2 Suppl):3S–21S. - PubMed
-
- Davis J. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Hospital Report. 1910;15:307–310.
LinkOut - more resources
Full Text Sources
Other Literature Sources
