Defective cholesterol metabolism in amyotrophic lateral sclerosis
- PMID: 27811233
- PMCID: PMC5234729
- DOI: 10.1194/jlr.P071639
Defective cholesterol metabolism in amyotrophic lateral sclerosis
Abstract
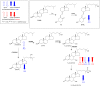
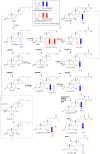
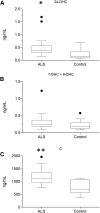
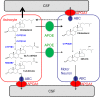
As neurons die, cholesterol is released in the central nervous system (CNS); hence, this sterol and its metabolites may represent a biomarker of neurodegeneration, including in amyotrophic lateral sclerosis (ALS), in which altered cholesterol levels have been linked to prognosis. More than 40 different sterols were quantified in serum and cerebrospinal fluid (CSF) from ALS patients and healthy controls. In CSF, the concentration of cholesterol was found to be elevated in ALS samples. When CSF metabolite levels were normalized to cholesterol, the cholesterol metabolite 3β,7α-dihydroxycholest-5-en-26-oic acid, along with its precursor 3β-hydroxycholest-5-en-26-oic acid and product 7α-hydroxy-3-oxocholest-4-en-26-oic acid, were reduced in concentration, whereas metabolites known to be imported from the circulation into the CNS were not found to differ in concentration between groups. Analysis of serum revealed that (25R)26-hydroxycholesterol, the immediate precursor of 3β-hydroxycholest-5-en-26-oic acid, was reduced in concentration in ALS patients compared with controls. We conclude that the acidic branch of bile acid biosynthesis, known to be operative in-part in the brain, is defective in ALS, leading to a failure of the CNS to remove excess cholesterol, which may be toxic to neuronal cells, compounded by a reduction in neuroprotective 3β,7α-dihydroxycholest-5-en-26-oic acid.
Keywords: bile acids and salts/biosynthesis; brain lipids; cholestenoic acids; cytochrome P450; mass spectrometry; neurodeneneration.; nuclear receptors/LXR; oxysterols.
Copyright © 2017 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
Cerebrospinal fluid steroidomics: are bioactive bile acids present in brain?J Biol Chem. 2010 Feb 12;285(7):4666-79. doi: 10.1074/jbc.M109.086678. Epub 2009 Dec 7. J Biol Chem. 2010. PMID: 19996111 Free PMC article.
-
Reduced Plasma Levels of 25-Hydroxycholesterol and Increased Cerebrospinal Fluid Levels of Bile Acid Precursors in Multiple Sclerosis Patients.Mol Neurobiol. 2017 Dec;54(10):8009-8020. doi: 10.1007/s12035-016-0281-9. Epub 2016 Nov 23. Mol Neurobiol. 2017. PMID: 27878760 Free PMC article.
-
Additional pathways of sterol metabolism: Evidence from analysis of Cyp27a1-/- mouse brain and plasma.Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Feb;1864(2):191-211. doi: 10.1016/j.bbalip.2018.11.006. Epub 2018 Nov 22. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. PMID: 30471425 Free PMC article.
-
Cholesterol dyshomeostasis in amyotrophic lateral sclerosis: cause, consequence, or epiphenomenon?FEBS J. 2022 Dec;289(24):7688-7709. doi: 10.1111/febs.16175. Epub 2021 Sep 14. FEBS J. 2022. PMID: 34469619 Review.
-
Neurofilaments as Biomarkers for Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis.PLoS One. 2016 Oct 12;11(10):e0164625. doi: 10.1371/journal.pone.0164625. eCollection 2016. PLoS One. 2016. PMID: 27732645 Free PMC article. Review.
Cited by
-
Primary care blood tests show lipid profile changes in pre-symptomatic amyotrophic lateral sclerosis.Brain Commun. 2023 Jul 28;5(4):fcad211. doi: 10.1093/braincomms/fcad211. eCollection 2023. Brain Commun. 2023. PMID: 37577380 Free PMC article.
-
Bile Acids Induce Neurite Outgrowth in Nsc-34 Cells via TGR5 and a Distinct Transcriptional Profile.Pharmaceuticals (Basel). 2023 Jan 24;16(2):174. doi: 10.3390/ph16020174. Pharmaceuticals (Basel). 2023. PMID: 37259326 Free PMC article.
-
Amyotrophic Lateral Sclerosis (ALS): Stressed by Dysfunctional Mitochondria-Endoplasmic Reticulum Contacts (MERCs).Cells. 2021 Jul 15;10(7):1789. doi: 10.3390/cells10071789. Cells. 2021. PMID: 34359958 Free PMC article. Review.
-
Endoplasmic Reticulum Malfunction in the Nervous System.Front Neurosci. 2017 Apr 25;11:220. doi: 10.3389/fnins.2017.00220. eCollection 2017. Front Neurosci. 2017. PMID: 28487627 Free PMC article.
-
Evaluation of the Hematological and Serum Biochemistry Parameters in the Pre-Symptomatic and Symptomatic Stages of ALS Disease to Support Early Diagnosis and Prognosis.Cells. 2022 Nov 11;11(22):3569. doi: 10.3390/cells11223569. Cells. 2022. PMID: 36428998 Free PMC article.
References
-
- Kiernan M. C., Vucic S., Cheah B. C., Turner M. R., Eisen A., Hardiman O., Burrell J. R., and Zoing M. C.. 2011. Amyotrophic lateral sclerosis. Lancet. 377: 942–955. - PubMed
-
- Kumar A., Bala L., Kalita J., Misra U. K., Singh R. L., Khetrapal C. L., and Babu G. N.. 2010. Metabolomic analysis of serum by (1) H NMR spectroscopy in amyotrophic lateral sclerosis. Clin. Chim. Acta. 411: 563–567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MR/K01014X/1/MRC_/Medical Research Council/United Kingdom
- TURNER/JAN13/944-795/MNDA_/Motor Neurone Disease Association/United Kingdom
- TURNER/NOV07/6501/MNDA_/Motor Neurone Disease Association/United Kingdom
- BB/I001735/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0701923/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

