Secreted NS1 Protects Dengue Virus from Mannose-Binding Lectin-Mediated Neutralization
- PMID: 27798151
- PMCID: PMC5123808
- DOI: 10.4049/jimmunol.1600323
Secreted NS1 Protects Dengue Virus from Mannose-Binding Lectin-Mediated Neutralization
Abstract
Flavivirus nonstructural protein 1 (NS1) is a unique secreted nonstructural glycoprotein. Although it is absent from the flavivirus virion, intracellular and extracellular forms of NS1 have essential roles in viral replication and the pathogenesis of infection. The fate of NS1 in insect cells has been more controversial, with some reports suggesting it is exclusively cell associated. In this study, we confirm NS1 secretion from cells of insect origin and characterize its physical, biochemical, and functional properties in the context of dengue virus (DENV) infection. Unlike mammalian cell-derived NS1, which displays both high mannose and complex type N-linked glycans, soluble NS1 secreted from DENV-infected insect cells contains only high mannose glycans. Insect cell-derived secreted NS1 also has different physical properties, including smaller and more heterogeneous sizes and the formation of less stable NS1 hexamers. Both mammalian and insect cell-derived NS1 bind to complement proteins C1s, C4, and C4-binding protein, as well as to a novel partner, mannose-binding lectin. Binding of NS1 to MBL protects DENV against mannose-binding lectin-mediated neutralization by the lectin pathway of complement activation. As we detected secreted NS1 and DENV together in the saliva of infected Aedes aegypti mosquitoes, these findings suggest a mechanism of viral immune evasion at the very earliest phase of infection.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures

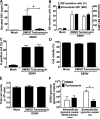

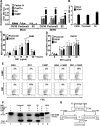
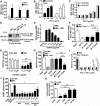
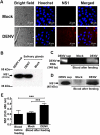
Similar articles
-
Dengue and the Lectin Pathway of the Complement System.Viruses. 2021 Jun 24;13(7):1219. doi: 10.3390/v13071219. Viruses. 2021. PMID: 34202570 Free PMC article. Review.
-
Complement-mediated neutralization of dengue virus requires mannose-binding lectin.mBio. 2011 Dec 13;2(6):e00276-11. doi: 10.1128/mBio.00276-11. Print 2011. mBio. 2011. PMID: 22167226 Free PMC article.
-
Inhibition of the Membrane Attack Complex by Dengue Virus NS1 through Interaction with Vitronectin and Terminal Complement Proteins.J Virol. 2016 Oct 14;90(21):9570-9581. doi: 10.1128/JVI.00912-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27512066 Free PMC article.
-
Antagonism of the complement component C4 by flavivirus nonstructural protein NS1.J Exp Med. 2010 Apr 12;207(4):793-806. doi: 10.1084/jem.20092545. Epub 2010 Mar 22. J Exp Med. 2010. PMID: 20308361 Free PMC article.
-
The Good, the Bad, and the Shocking: The Multiple Roles of Dengue Virus Nonstructural Protein 1 in Protection and Pathogenesis.Annu Rev Virol. 2018 Sep 29;5(1):227-253. doi: 10.1146/annurev-virology-101416-041848. Epub 2018 Jul 25. Annu Rev Virol. 2018. PMID: 30044715 Free PMC article. Review.
Cited by
-
The N and C-terminal deleted variant of the dengue virus NS1 protein is a potential candidate for dengue vaccine development.Sci Rep. 2024 Aug 14;14(1):18883. doi: 10.1038/s41598-024-65593-1. Sci Rep. 2024. PMID: 39143088 Free PMC article.
-
The Dengue Virus Nonstructural Protein 1 (NS1) Is Secreted from Mosquito Cells in Association with the Intracellular Cholesterol Transporter Chaperone Caveolin Complex.J Virol. 2019 Feb 5;93(4):e01985-18. doi: 10.1128/JVI.01985-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463973 Free PMC article.
-
Role of Host-Mediated Post-Translational Modifications (PTMs) in RNA Virus Pathogenesis.Int J Mol Sci. 2020 Dec 30;22(1):323. doi: 10.3390/ijms22010323. Int J Mol Sci. 2020. PMID: 33396899 Free PMC article. Review.
-
Let's Get Physical: Flavivirus-Host Protein-Protein Interactions in Replication and Pathogenesis.Front Microbiol. 2022 Mar 3;13:847588. doi: 10.3389/fmicb.2022.847588. eCollection 2022. Front Microbiol. 2022. PMID: 35308381 Free PMC article. Review.
-
Complement-Mediated Neutralisation Identified in Ebola Virus Disease Survivor Plasma: Implications for Protection and Pathogenesis.Front Immunol. 2022 Apr 12;13:857481. doi: 10.3389/fimmu.2022.857481. eCollection 2022. Front Immunol. 2022. PMID: 35493467 Free PMC article.
References
-
- WHO . Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Haemorrhagic Fever, Revised and expanded edition ed. WHO Library Cataloguing-in-Publication data, World Health Organization, Regional Office for South-East Asia; 2011.
-
- Brandt WE, Chiewslip D, Harris DL, Russell PK. Partial purification and characterization of a dengue virus soluble complement-fixing antigen. J Immunol. 1970;105:1565–1568. - PubMed
-
- Muller DA, Young PR. The flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antiviral Res. 2013;98:192–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

