A novel fission-independent role of dynamin-related protein 1 in cardiac mitochondrial respiration
- PMID: 27794519
- PMCID: PMC5340145
- DOI: 10.1093/cvr/cvw212
A novel fission-independent role of dynamin-related protein 1 in cardiac mitochondrial respiration
Abstract
Aims: Mitochondria in adult cardiomyocytes exhibit static morphology and infrequent dynamic changes, despite the high abundance of fission and fusion regulatory proteins in the heart. Previous reports have indicated that fusion proteins may bear functions beyond morphology regulation. Here, we investigated the role of fission protein, dynamin-related protein 1 (DRP1), on mitochondrial respiration regulation in adult cardiomyocytes.
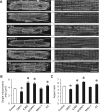
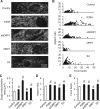
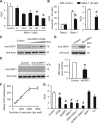
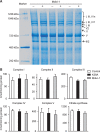
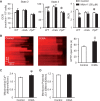
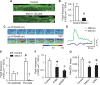
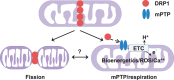
Methods and results: By using genetic or pharmacological approaches, we manipulated the activity or protein level of fission and fusion proteins and found they mildly influenced mitochondrial morphology in adult rodent cardiomyocytes, which is in contrast to their significant effect in H9C2 cardiac myoblasts. Intriguingly, inhibiting endogenous DRP1 by dominant-negative DRP1 mutation (K38A), shRNA, or Mdivi-1 suppressed maximal respiration and respiratory control ratio in isolated mitochondria from adult mouse heart or in adult cardiomyocytes from rat. Meanwhile, basal respiration was increased due to increased proton leak. Facilitating mitofusin-mediated fusion by S3 compound, however, failed to inhibit mitochondrial respiration in adult cardiomyocytes. Mechanistically, DRP1 inhibition did not affect the maximal activity of individual respiratory chain complexes or the assembly of supercomplexes. Knocking out cyclophilin D, a regulator of mitochondrial permeability transition pore (mPTP), abolished the effect of DRP1 inhibition on respiration. Finally, DRP1 inhibition decreased transient mPTP-mediated mitochondrial flashes, delayed laser-induced mPTP opening and suppressed mitochondrial reactive oxygen species (ROS).
Conclusion: These results uncover a novel non-canonical function of the fission protein, DRP1 in maintaining or positively stimulating mitochondrial respiration, bioenergetics and ROS signalling in adult cardiomyocyte, which is likely independent of morphological changes.
Keywords: Adult cardiomyocyte; Dynamin related protein 1; Mitochondrial morphology; Mitochondrial permeability transition pore; Mitochondrial respiration.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2016. For Permissions, please email: journals.permissions@oup.com.
Figures
Comment in
-
A new side to an old coin: dynamin related protein-1 with benefits in the heart.Cardiovasc Res. 2017 Feb;113(2):118-119. doi: 10.1093/cvr/cvw255. Epub 2016 Dec 21. Cardiovasc Res. 2017. PMID: 28003271 No abstract available.
Similar articles
-
Inhibition of dynamin-related protein 1 protects against myocardial ischemia-reperfusion injury in diabetic mice.Cardiovasc Diabetol. 2017 Feb 7;16(1):19. doi: 10.1186/s12933-017-0501-2. Cardiovasc Diabetol. 2017. PMID: 28173848 Free PMC article.
-
Protein kinase D activation induces mitochondrial fragmentation and dysfunction in cardiomyocytes.J Physiol. 2018 Mar 1;596(5):827-855. doi: 10.1113/JP275418. Epub 2018 Jan 25. J Physiol. 2018. PMID: 29313986 Free PMC article.
-
Inhibition of miR-23a attenuates doxorubicin-induced mitochondria-dependent cardiomyocyte apoptosis by targeting the PGC-1α/Drp1 pathway.Toxicol Appl Pharmacol. 2019 Apr 15;369:73-81. doi: 10.1016/j.taap.2019.02.016. Epub 2019 Mar 1. Toxicol Appl Pharmacol. 2019. PMID: 30831132
-
Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore.Circ J. 2013;77(5):1111-22. doi: 10.1253/circj.cj-13-0321. Epub 2013 Mar 29. Circ J. 2013. PMID: 23538482 Free PMC article. Review.
-
The role of Drp1 in mitophagy and cell death in the heart.J Mol Cell Cardiol. 2020 May;142:138-145. doi: 10.1016/j.yjmcc.2020.04.015. Epub 2020 Apr 14. J Mol Cell Cardiol. 2020. PMID: 32302592 Free PMC article. Review.
Cited by
-
Detailed analysis of Mdivi-1 effects on complex I and respiratory supercomplex assembly.Sci Rep. 2024 Aug 24;14(1):19673. doi: 10.1038/s41598-024-69748-y. Sci Rep. 2024. PMID: 39187541 Free PMC article.
-
Reduction of elevated proton leak rejuvenates mitochondria in the aged cardiomyocyte.Elife. 2020 Dec 15;9:e60827. doi: 10.7554/eLife.60827. Elife. 2020. PMID: 33319746 Free PMC article.
-
Hydralazine protects the heart against acute ischaemia/reperfusion injury by inhibiting Drp1-mediated mitochondrial fission.Cardiovasc Res. 2022 Jan 7;118(1):282-294. doi: 10.1093/cvr/cvaa343. Cardiovasc Res. 2022. PMID: 33386841 Free PMC article.
-
Why don't mice lacking the mitochondrial Ca2+ uniporter experience an energy crisis?J Physiol. 2020 Apr;598(7):1307-1326. doi: 10.1113/JP276636. Epub 2018 Oct 11. J Physiol. 2020. PMID: 30218574 Free PMC article. Review.
-
Elongated mitochondrial constrictions and fission in muscle fatigue.J Cell Sci. 2018 Dec 5;131(23):jcs221028. doi: 10.1242/jcs.221028. J Cell Sci. 2018. PMID: 30404834 Free PMC article.
References
-
- Scheffler IE. Mitochondria. J. New York, NY USA: Wiley and Sons, Inc; 2008.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

