Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation
- PMID: 27793571
- PMCID: PMC5205568
- DOI: 10.1016/j.it.2016.10.001
Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation
Abstract
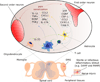
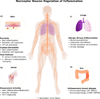
Nociceptor sensory neurons protect organisms from danger by eliciting pain and driving avoidance. Pain also accompanies many types of inflammation and injury. It is increasingly clear that active crosstalk occurs between nociceptor neurons and the immune system to regulate pain, host defense, and inflammatory diseases. Immune cells at peripheral nerve terminals and within the spinal cord release mediators that modulate mechanical and thermal sensitivity. In turn, nociceptor neurons release neuropeptides and neurotransmitters from nerve terminals that regulate vascular, innate, and adaptive immune cell responses. Therefore, the dialog between nociceptor neurons and the immune system is a fundamental aspect of inflammation, both acute and chronic. A better understanding of these interactions could produce approaches to treat chronic pain and inflammatory diseases.
Keywords: inflammation; neuroimmunology; nociceptor; pain; sensory neuron.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
The role of neutrophils in neuro-immune modulation.Pharmacol Res. 2020 Jan;151:104580. doi: 10.1016/j.phrs.2019.104580. Epub 2019 Nov 28. Pharmacol Res. 2020. PMID: 31786317 Free PMC article. Review.
-
Pain and immunity: implications for host defence.Nat Rev Immunol. 2019 Jul;19(7):433-447. doi: 10.1038/s41577-019-0147-2. Nat Rev Immunol. 2019. PMID: 30874629 Free PMC article. Review.
-
Cutaneous Neuroimmune Interactions in Peripheral Neuropathic Pain States.Front Immunol. 2021 Apr 12;12:660203. doi: 10.3389/fimmu.2021.660203. eCollection 2021. Front Immunol. 2021. PMID: 33912189 Free PMC article. Review.
-
Teasing Out the Interplay Between Natural Killer Cells and Nociceptor Neurons.J Vis Exp. 2022 Jun 30;(184). doi: 10.3791/63800. J Vis Exp. 2022. PMID: 35848838
-
Profiling of how nociceptor neurons detect danger - new and old foes.J Intern Med. 2019 Sep;286(3):268-289. doi: 10.1111/joim.12957. Epub 2019 Jul 29. J Intern Med. 2019. PMID: 31282104 Review.
Cited by
-
The influence of sex on neuroimmune communication, pain, and physiology.Biol Sex Differ. 2024 Oct 22;15(1):82. doi: 10.1186/s13293-024-00660-w. Biol Sex Differ. 2024. PMID: 39439003 Free PMC article. Review.
-
GABA signaling enforces intestinal germinal center B cell differentiation.Proc Natl Acad Sci U S A. 2022 Nov;119(44):e2215921119. doi: 10.1073/pnas.2215921119. Epub 2022 Oct 24. Proc Natl Acad Sci U S A. 2022. PMID: 36279432 Free PMC article.
-
The role of neuroinflammation in the transition of acute to chronic pain and the opioid-induced hyperalgesia and tolerance.Front Pharmacol. 2023 Dec 15;14:1297931. doi: 10.3389/fphar.2023.1297931. eCollection 2023. Front Pharmacol. 2023. PMID: 38161698 Free PMC article. Review.
-
Inflammatory pain resolution by mouse serum-derived small extracellular vesicles.bioRxiv [Preprint]. 2024 Feb 18:2024.02.16.578759. doi: 10.1101/2024.02.16.578759. bioRxiv. 2024. Update in: Brain Behav Immun. 2025 Jan;123:422-441. doi: 10.1016/j.bbi.2024.09.032 PMID: 38405813 Free PMC article. Updated. Preprint.
-
Oral Supplementation with Ultramicronized Palmitoylethanolamide for Joint Disease and Lameness Management in Four Jumping Horses: A Case Report.Animals (Basel). 2020 Aug 21;10(9):1469. doi: 10.3390/ani10091469. Animals (Basel). 2020. PMID: 32825646 Free PMC article.
References
-
- Wood JN, et al. Voltage-gated sodium channels and pain pathways. J. Neurobiol. 2004;61:55–71. - PubMed
-
- Cunha TM, et al. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J. Leukoc. Biol. 2008;83:824–832. - PubMed
-
- Kiguchi N, et al. Epigenetic Augmentation of the Macrophage Inflammatory Protein 2/C-X-C Chemokine Receptor Type 2 Axis through Histone H3 Acetylation in Injured Peripheral Nerves Elicits Neuropathic Pain. J. Pharmacol. Exp. Ther. 2012;340:577–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

