Regulation of mRNA capping in the cell cycle
- PMID: 27791484
- PMCID: PMC5270520
- DOI: 10.1080/15476286.2016.1251540
Regulation of mRNA capping in the cell cycle
Abstract
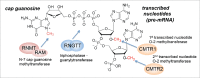
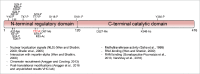
The mRNA cap structure, which is added to nascent RNA pol II transcripts, recruits the protein complexes required for pre-mRNA transcript processing, mRNA export and translation initiation. The enzymes which catalyze mRNA cap synthesis are regulated by cellular signaling pathways which impact on their expression, localization and activity. Here we discuss the recent observation that the mRNA cap methyltransferase, RNMT, is phosphorylated on Thr-77 by CDK1-cyclin B1, which regulates its activity and the proteins with which it interacts. RNMT Thr-77 phosphorylation provides a burst of mRNA cap methyltransferase activity during early G1 phase at a time when transcription is reactivated following completion of the cell cycle. This co-ordination of transcription and mRNA capping makes an important contribution to gene expression in the cell; preventing RNMT Thr-77 phosphorylation inhibits cell proliferation. Here we discuss these findings and how mRNA cap synthesis may be regulated in other scenarios.
Keywords: Cell cycle; RNMT; gene expression; mRNA; mRNA cap; phosphorylation; transcription; translation.
Figures
Similar articles
-
CDK1-Cyclin B1 Activates RNMT, Coordinating mRNA Cap Methylation with G1 Phase Transcription.Mol Cell. 2016 Mar 3;61(5):734-746. doi: 10.1016/j.molcel.2016.02.008. Mol Cell. 2016. PMID: 26942677 Free PMC article.
-
c-Myc co-ordinates mRNA cap methylation and ribosomal RNA production.Biochem J. 2017 Feb 1;474(3):377-384. doi: 10.1042/BCJ20160930. Epub 2016 Dec 1. Biochem J. 2017. PMID: 27934633 Free PMC article.
-
mRNA Cap Methyltransferase, RNMT-RAM, Promotes RNA Pol II-Dependent Transcription.Cell Rep. 2018 May 1;23(5):1530-1542. doi: 10.1016/j.celrep.2018.04.004. Cell Rep. 2018. PMID: 29719263 Free PMC article.
-
The RNA cap methyltransferases RNMT and CMTR1 co-ordinate gene expression during neural differentiation.Biochem Soc Trans. 2023 Jun 28;51(3):1131-1141. doi: 10.1042/BST20221154. Biochem Soc Trans. 2023. PMID: 37145036 Free PMC article. Review.
-
Myc and mRNA capping.Biochim Biophys Acta. 2015 May;1849(5):501-5. doi: 10.1016/j.bbagrm.2014.03.007. Epub 2014 Mar 27. Biochim Biophys Acta. 2015. PMID: 24681440 Free PMC article. Review.
Cited by
-
RNA-binding proteins and heat-shock protein 90 are constituents of the cytoplasmic capping enzyme interactome.J Biol Chem. 2018 Oct 26;293(43):16596-16607. doi: 10.1074/jbc.RA118.004973. Epub 2018 Aug 30. J Biol Chem. 2018. PMID: 30166341 Free PMC article.
-
RNA-modifying enzymes and their function in a chromatin context.Nat Struct Mol Biol. 2019 Oct;26(10):858-862. doi: 10.1038/s41594-019-0312-0. Epub 2019 Oct 3. Nat Struct Mol Biol. 2019. PMID: 31582848 Free PMC article. Review.
-
Interplay of mRNA capping and transcription machineries.Biosci Rep. 2020 Jan 31;40(1):BSR20192825. doi: 10.1042/BSR20192825. Biosci Rep. 2020. PMID: 31904821 Free PMC article. Review.
-
A recap of RNA recapping.Wiley Interdiscip Rev RNA. 2019 Jan;10(1):e1504. doi: 10.1002/wrna.1504. Epub 2018 Sep 5. Wiley Interdiscip Rev RNA. 2019. PMID: 30252202 Free PMC article. Review.
-
RNA guanine-7 methyltransferase catalyzes the methylation of cytoplasmically recapped RNAs.Nucleic Acids Res. 2017 Oct 13;45(18):10726-10739. doi: 10.1093/nar/gkx801. Nucleic Acids Res. 2017. PMID: 28981715 Free PMC article.
References
-
- Shuman S. RNA capping: progress and prospects. Rna 2015; 21:735-7; PMID:25780214; http://dx.doi.org/10.1261/rna.049973.115 - DOI - PMC - PubMed
-
- Shatkin AJ. Capping of eucaryotic mRNAs. Cell 1976; 9:645-53; PMID:1017010; http://dx.doi.org/10.1016/0092-8674(76)90128-8 - DOI - PubMed
-
- Furuichi Y. Discovery of m(7)G-cap in eukaryotic mRNAs. Proc Jpn Acad Ser B Phys Biol Sci 2015; 91:394-409; PMID:26460318; http://dx.doi.org/10.2183/pjab.91.394 - DOI - PMC - PubMed
-
- Ramanathan A, Robb GB, Chan SH. mRNA capping: biological functions and applications. Nucleic Acids Res 2016; 44(16):7511-26; PMID:27317694; http://dx.doi.org/2195701010.1093/nar/gkw551 - DOI - PMC - PubMed
-
- Topisirovic I, Svitkin YV, Sonenberg N, Shatkin AJ. Cap and cap-binding proteins in the control of gene expression. Wiley Interdiscip Rev RNA 2011; 2:277-98; PMID:21957010; http://dx.doi.org/10.1002/wrna.52 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
