Nox-2-Mediated Phenotype Loss of Hippocampal Parvalbumin Interneurons Might Contribute to Postoperative Cognitive Decline in Aging Mice
- PMID: 27790135
- PMCID: PMC5062642
- DOI: 10.3389/fnagi.2016.00234
Nox-2-Mediated Phenotype Loss of Hippocampal Parvalbumin Interneurons Might Contribute to Postoperative Cognitive Decline in Aging Mice
Abstract
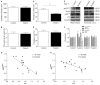
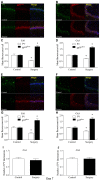
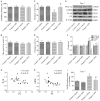
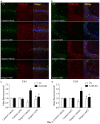
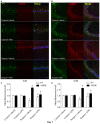
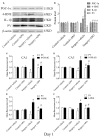
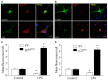
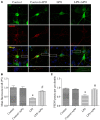
Postoperative cognitive decline (POCD) is a common complication following anesthesia and surgery, especially in elderly patients; however, the precise mechanisms of POCD remain unclear. Here, we investigated whether nicotinamide adenine dinucleotide phosphate (NADPH) oxidase mediated-abnormalities in parvalbumin (PV) interneurons play an important role in the pathophysiology of POCD. The animal model was established using isoflurane anesthesia and exploratory laparotomy in 16-month-old male C57BL/6 mice. For interventional experiments, mice were chronically treated with the NADPH oxidase inhibitor apocynin (APO). Open field and fear conditioning behavioral tests were performed on day 6 and 7 post-surgery, respectively. In a separate experiment, brain tissue was harvested and subjected to biochemical analysis. Primary hippocampal neurons challenged with lipopolysaccharide (LPS) in vitro were used to investigate the mechanisms underlying the oxidative stress-induced abnormalities in PV interneurons. Our results showed that anesthesia and surgery induced significant hippocampus-dependent memory impairment, which was accompanied by PV interneuron phenotype loss and increased expression of interleukin-1β (IL-1β), markers of oxidative stress and NADPH oxidase 2 (Nox2) in the hippocampus. In addition, LPS exposure increased Nox2 level and decreased the expression of PV and the number of excitatory synapses onto PV interneurons in the primary hippocampal neurons. Notably, treatment with APO reversed these abnormalities. Our study suggests that Nox2-derived reactive oxygen species (ROS) production triggers, at least in part, anesthesia- and surgery-induced hippocampal PV interneuron phenotype loss and consequent cognitive impairment in aging mice.
Keywords: Nox2; aging; apocynin; hippocampus; parvalbumin; postoperative cognitive decline.
Figures

Similar articles
-
Sepsis-induced selective parvalbumin interneuron phenotype loss and cognitive impairments may be mediated by NADPH oxidase 2 activation in mice.J Neuroinflammation. 2015 Sep 29;12:182. doi: 10.1186/s12974-015-0401-x. J Neuroinflammation. 2015. PMID: 26416717 Free PMC article.
-
NADPH oxidase 2-derived reactive oxygen species in the hippocampus might contribute to microglial activation in postoperative cognitive dysfunction in aged mice.Brain Behav Immun. 2016 Jan;51:109-118. doi: 10.1016/j.bbi.2015.08.002. Epub 2015 Aug 4. Brain Behav Immun. 2016. PMID: 26254234
-
NOX2 Mediated-Parvalbumin Interneuron Loss Might Contribute to Anxiety-Like and Enhanced Fear Learning Behavior in a Rat Model of Post-Traumatic Stress Disorder.Mol Neurobiol. 2016 Dec;53(10):6680-6689. doi: 10.1007/s12035-015-9571-x. Epub 2015 Dec 9. Mol Neurobiol. 2016. PMID: 26650043
-
Amelioration of oxidative stress-induced phenotype loss of parvalbumin interneurons might contribute to the beneficial effects of environmental enrichment in a rat model of post-traumatic stress disorder.Behav Brain Res. 2016 Oct 1;312:84-92. doi: 10.1016/j.bbr.2016.06.016. Epub 2016 Jun 11. Behav Brain Res. 2016. PMID: 27297027
-
Reactive Oxygen Species-mediated Loss of Phenotype of Parvalbumin Interneurons Contributes to Long-term Cognitive Impairments After Repeated Neonatal Ketamine Exposures.Neurotox Res. 2016 Nov;30(4):593-605. doi: 10.1007/s12640-016-9653-1. Epub 2016 Jul 21. Neurotox Res. 2016. PMID: 27443555
Cited by
-
SynCAM1 deficiency in the hippocampal parvalbumin interneurons contributes to sevoflurane-induced cognitive impairment in neonatal rats.CNS Neurosci Ther. 2024 Jan;30(1):e14554. doi: 10.1111/cns.14554. Epub 2023 Dec 17. CNS Neurosci Ther. 2024. PMID: 38105652 Free PMC article.
-
PGE2-EP3 signaling exacerbates hippocampus-dependent cognitive impairment after laparotomy by reducing expression levels of hippocampal synaptic plasticity-related proteins in aged mice.CNS Neurosci Ther. 2018 Oct;24(10):917-929. doi: 10.1111/cns.12832. Epub 2018 Feb 27. CNS Neurosci Ther. 2018. PMID: 29488342 Free PMC article.
-
Transient neuroinflammation following surgery contributes to long-lasting cognitive decline in elderly rats via dysfunction of synaptic NMDA receptor.J Neuroinflammation. 2022 Jul 13;19(1):181. doi: 10.1186/s12974-022-02528-5. J Neuroinflammation. 2022. PMID: 35831873 Free PMC article.
-
Relevance of Cortical and Hippocampal Interneuron Functional Diversity to General Anesthetic Mechanisms: A Narrative Review.Front Synaptic Neurosci. 2022 Jan 26;13:812905. doi: 10.3389/fnsyn.2021.812905. eCollection 2021. Front Synaptic Neurosci. 2022. PMID: 35153712 Free PMC article. Review.
-
Dysregulation of BDNF/TrkB signaling mediated by NMDAR/Ca2+/calpain might contribute to postoperative cognitive dysfunction in aging mice.J Neuroinflammation. 2020 Jan 16;17(1):23. doi: 10.1186/s12974-019-1695-x. J Neuroinflammation. 2020. PMID: 31948437 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

