Modulation of mitochondrial function by the microbiome metabolite propionic acid in autism and control cell lines
- PMID: 27779624
- PMCID: PMC5290345
- DOI: 10.1038/tp.2016.189
Modulation of mitochondrial function by the microbiome metabolite propionic acid in autism and control cell lines
Abstract
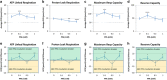
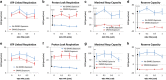
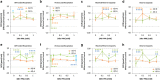
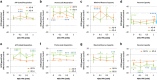
Propionic acid (PPA) is a ubiquitous short-chain fatty acid, which is a major fermentation product of the enteric microbiome. PPA is a normal intermediate of metabolism and is found in foods, either naturally or as a preservative. PPA and its derivatives have been implicated in both health and disease. Whereas PPA is an energy substrate and has many proposed beneficial effects, it is also associated with human disorders involving mitochondrial dysfunction, including propionic acidemia and autism spectrum disorders (ASDs). We aimed to investigate the dichotomy between the health and disease effects of PPA by measuring mitochondrial function in ASD and age- and gender-matched control lymphoblastoid cell lines (LCLs) following incubation with PPA at several concentrations and durations both with and without an in vitro increase in reactive oxygen species (ROS). Mitochondrial function was optimally increased at particular exposure durations and concentrations of PPA with ASD LCLs, demonstrating a greater enhancement. In contrast, increasing ROS negated the positive PPA effect with the ASD LCLs, showing a greater detriment. These data demonstrate that enteric microbiome metabolites such as PPA can have both beneficial and toxic effects on mitochondrial function, depending on concentration, exposure duration and microenvironment redox state with these effects amplified in LCLs derived from individuals with ASD. As PPA, as well as enteric bacteria, which produce PPA, have been implicated in a wide variety of diseases, including ASD, diabetes, obesity and inflammatory diseases, insight into this metabolic modulator from the host microbiome may have wide applications for both health and disease.
Figures

Similar articles
-
Modulation of Immunological Pathways in Autistic and Neurotypical Lymphoblastoid Cell Lines by the Enteric Microbiome Metabolite Propionic Acid.Front Immunol. 2017 Dec 22;8:1670. doi: 10.3389/fimmu.2017.01670. eCollection 2017. Front Immunol. 2017. PMID: 29312285 Free PMC article.
-
Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism.Transl Psychiatry. 2018 Feb 2;8(1):42. doi: 10.1038/s41398-017-0089-z. Transl Psychiatry. 2018. PMID: 29391397 Free PMC article.
-
Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder.Transl Psychiatry. 2013 Jan 22;3(1):e220. doi: 10.1038/tp.2012.143. Transl Psychiatry. 2013. PMID: 23340503 Free PMC article.
-
Mitochondrial function and abnormalities implicated in the pathogenesis of ASD.Prog Neuropsychopharmacol Biol Psychiatry. 2019 Jun 8;92:83-108. doi: 10.1016/j.pnpbp.2018.12.015. Epub 2018 Dec 29. Prog Neuropsychopharmacol Biol Psychiatry. 2019. PMID: 30599156 Review.
-
Pathophysiology of propionic and methylmalonic acidemias. Part 1: Complications.J Inherit Metab Dis. 2019 Sep;42(5):730-744. doi: 10.1002/jimd.12129. Epub 2019 Aug 7. J Inherit Metab Dis. 2019. PMID: 31119747 Review.
Cited by
-
Prenatal air pollution influences neurodevelopment and behavior in autism spectrum disorder by modulating mitochondrial physiology.Mol Psychiatry. 2021 May;26(5):1561-1577. doi: 10.1038/s41380-020-00885-2. Epub 2020 Sep 22. Mol Psychiatry. 2021. PMID: 32963337 Free PMC article.
-
Clinical and Molecular Characteristics of Mitochondrial Dysfunction in Autism Spectrum Disorder.Mol Diagn Ther. 2018 Oct;22(5):571-593. doi: 10.1007/s40291-018-0352-x. Mol Diagn Ther. 2018. PMID: 30039193 Free PMC article. Review.
-
The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder.Int J Mol Sci. 2019 Apr 29;20(9):2115. doi: 10.3390/ijms20092115. Int J Mol Sci. 2019. PMID: 31035684 Free PMC article. Review.
-
Autistic Siblings with Novel Mutations in Two Different Genes: Insight for Genetic Workups of Autistic Siblings and Connection to Mitochondrial Dysfunction.Front Pediatr. 2017 Oct 12;5:219. doi: 10.3389/fped.2017.00219. eCollection 2017. Front Pediatr. 2017. PMID: 29075622 Free PMC article.
-
Central Nervous System Metabolism in Autism, Epilepsy and Developmental Delays: A Cerebrospinal Fluid Analysis.Metabolites. 2022 Apr 20;12(5):371. doi: 10.3390/metabo12050371. Metabolites. 2022. PMID: 35629876 Free PMC article.
References
-
- Sherwin E, Rea K, Dinan TG, Cryan JF. A gut (microbiome) feeling about the brain. Curr Opin Gastroenterol 2016; 32: 96–102. - PubMed
-
- Borre YE, Moloney RD, Clarke G, Dinan TG, Cryan JF. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv Exp Med Biol 2014; 817: 373–403. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

