Fetus-derived DLK1 is required for maternal metabolic adaptations to pregnancy and is associated with fetal growth restriction
- PMID: 27776119
- PMCID: PMC5373434
- DOI: 10.1038/ng.3699
Fetus-derived DLK1 is required for maternal metabolic adaptations to pregnancy and is associated with fetal growth restriction
Abstract
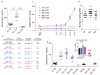
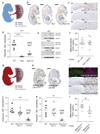
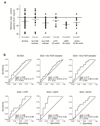
Pregnancy is a state of high metabolic demand. Fasting diverts metabolism to fatty acid oxidation, and the fasted response occurs much more rapidly in pregnant women than in non-pregnant women. The product of the imprinted DLK1 gene (delta-like homolog 1) is an endocrine signaling molecule that reaches a high concentration in the maternal circulation during late pregnancy. By using mouse models with deleted Dlk1, we show that the fetus is the source of maternal circulating DLK1. In the absence of fetally derived DLK1, the maternal fasting response is impaired. Furthermore, we found that maternal circulating DLK1 levels predict embryonic mass in mice and can differentiate healthy small-for-gestational-age (SGA) infants from pathologically small infants in a human cohort. Therefore, measurement of DLK1 concentration in maternal blood may be a valuable method for diagnosing human disorders associated with impaired DLK1 expression and to predict poor intrauterine growth and complications of pregnancy.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Comment in
-
Metabolism: DLK1 levels predict fetal growth restriction.Nat Rev Endocrinol. 2017 Jan;13(1):4. doi: 10.1038/nrendo.2016.183. Epub 2016 Nov 4. Nat Rev Endocrinol. 2017. PMID: 27811939 No abstract available.
Similar articles
-
Low Maternal DLK1 Levels at 26 Weeks Is Associated With Small for Gestational Age at Birth.Front Endocrinol (Lausanne). 2022 Feb 28;13:836731. doi: 10.3389/fendo.2022.836731. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35295988 Free PMC article.
-
DLK1: A Novel Biomarker of Placental Insufficiency in Stillbirth and Live Birth.Am J Perinatol. 2024 May;41(S 01):e221-e229. doi: 10.1055/a-1877-6191. Epub 2022 Jun 16. Am J Perinatol. 2024. PMID: 35709732
-
Circulating Delta-like homolog 1 (DLK1) at 36 weeks is correlated with birthweight and is of placental origin.Placenta. 2020 Feb;91:24-30. doi: 10.1016/j.placenta.2020.01.003. Epub 2020 Jan 8. Placenta. 2020. PMID: 32174303
-
Screening for fetal growth restriction using fetal biometry combined with maternal biomarkers.Am J Obstet Gynecol. 2018 Feb;218(2S):S725-S737. doi: 10.1016/j.ajog.2017.12.002. Epub 2017 Dec 22. Am J Obstet Gynecol. 2018. PMID: 29275822 Review.
-
Associations Between Fetal Imprinted Genes and Maternal Blood Pressure in Pregnancy.Hypertension. 2016 Dec;68(6):1459-1466. doi: 10.1161/HYPERTENSIONAHA.116.08261. Epub 2016 Oct 24. Hypertension. 2016. PMID: 27777362 Free PMC article. Review.
Cited by
-
Low Maternal DLK1 Levels at 26 Weeks Is Associated With Small for Gestational Age at Birth.Front Endocrinol (Lausanne). 2022 Feb 28;13:836731. doi: 10.3389/fendo.2022.836731. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35295988 Free PMC article.
-
Exome sequencing of Finnish isolates enhances rare-variant association power.Nature. 2019 Aug;572(7769):323-328. doi: 10.1038/s41586-019-1457-z. Epub 2019 Jul 31. Nature. 2019. PMID: 31367044 Free PMC article.
-
Mitigation of Fetal Radiation Injury from Mid-Gestation Total-body Irradiation by Maternal Administration of Mitochondrial-Targeted GS-Nitroxide JP4-039.Radiat Res. 2024 Sep 1;202(3):565-579. doi: 10.1667/RADE-24-00095.1. Radiat Res. 2024. PMID: 39074819
-
Pituitary Tumor-Transforming Gene 1/Delta like Non-Canonical Notch Ligand 1 Signaling in Chronic Liver Diseases.Int J Mol Sci. 2022 Jun 21;23(13):6897. doi: 10.3390/ijms23136897. Int J Mol Sci. 2022. PMID: 35805898 Free PMC article. Review.
-
The long non-coding RNA Meg3 mediates imprinted gene expression during stem cell differentiation.Nucleic Acids Res. 2024 Jun 24;52(11):6183-6200. doi: 10.1093/nar/gkae247. Nucleic Acids Res. 2024. PMID: 38613389 Free PMC article.
References
-
- Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr. 2000;71(5 Suppl):1256S–61S. - PubMed
-
- Metzger BE, et al. “Accelerated starvation” and the skipped breakfast in late normal pregnancy. Lancet. 1982;1(8272):588–92. - PubMed
-
- Takada S, et al. Delta-like and Gtl2 are reciprocally expressed, differentially methylated linked imprinted genes on mouse chromosome 12. Curr Biol. 2000;10(18):1135–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

