Mutations in CRADD Result in Reduced Caspase-2-Mediated Neuronal Apoptosis and Cause Megalencephaly with a Rare Lissencephaly Variant
- PMID: 27773430
- PMCID: PMC5097945
- DOI: 10.1016/j.ajhg.2016.09.010
Mutations in CRADD Result in Reduced Caspase-2-Mediated Neuronal Apoptosis and Cause Megalencephaly with a Rare Lissencephaly Variant
Abstract
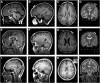
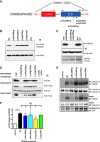
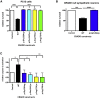
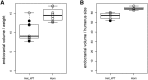
Lissencephaly is a malformation of cortical development typically caused by deficient neuronal migration resulting in cortical thickening and reduced gyration. Here we describe a "thin" lissencephaly (TLIS) variant characterized by megalencephaly, frontal predominant pachygyria, intellectual disability, and seizures. Trio-based whole-exome sequencing and targeted re-sequencing identified recessive mutations of CRADD in six individuals with TLIS from four unrelated families of diverse ethnic backgrounds. CRADD (also known as RAIDD) is a death-domain-containing adaptor protein that oligomerizes with PIDD and caspase-2 to initiate apoptosis. TLIS variants cluster in the CRADD death domain, a platform for interaction with other death-domain-containing proteins including PIDD. Although caspase-2 is expressed in the developing mammalian brain, little is known about its role in cortical development. CRADD/caspase-2 signaling is implicated in neurotrophic factor withdrawal- and amyloid-β-induced dendritic spine collapse and neuronal apoptosis, suggesting a role in cortical sculpting and plasticity. TLIS-associated CRADD variants do not disrupt interactions with caspase-2 or PIDD in co-immunoprecipitation assays, but still abolish CRADD's ability to activate caspase-2, resulting in reduced neuronal apoptosis in vitro. Homozygous Cradd knockout mice display megalencephaly and seizures without obvious defects in cortical lamination, supporting a role for CRADD/caspase-2 signaling in mammalian brain development. Megalencephaly and lissencephaly associated with defective programmed cell death from loss of CRADD function in humans implicate reduced apoptosis as an important pathophysiological mechanism of cortical malformation. Our data suggest that CRADD/caspase-2 signaling is critical for normal gyration of the developing human neocortex and for normal cognitive ability.
Keywords: MCD; apoptosis; epilepsy; intellectual disability; malformation of cortical development; mouse model; neurodevelopmental disorder; pachygyria.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
RAIDD mutations underlie the pathogenesis of thin lissencephaly (TLIS).PLoS One. 2018 Oct 3;13(10):e0205042. doi: 10.1371/journal.pone.0205042. eCollection 2018. PLoS One. 2018. PMID: 30281648 Free PMC article.
-
Homozygous null variant in CRADD, encoding an adaptor protein that mediates apoptosis, is associated with lissencephaly.Am J Med Genet A. 2017 Sep;173(9):2539-2544. doi: 10.1002/ajmg.a.38347. Epub 2017 Jul 7. Am J Med Genet A. 2017. PMID: 28686357
-
Phenotypic spectrum associated with a CRADD founder variant underlying frontotemporal predominant pachygyria in the Finnish population.Eur J Hum Genet. 2019 Aug;27(8):1235-1243. doi: 10.1038/s41431-019-0383-8. Epub 2019 Mar 26. Eur J Hum Genet. 2019. PMID: 30914828 Free PMC article.
-
Total recall: the role of PIDDosome components in neurodegeneration.Trends Mol Med. 2023 Dec;29(12):996-1013. doi: 10.1016/j.molmed.2023.08.008. Epub 2023 Sep 14. Trends Mol Med. 2023. PMID: 37716905 Review.
-
Are promyelocytic leukaemia protein nuclear bodies a scaffold for caspase-2 programmed cell death?Trends Biochem Sci. 2007 Sep;32(9):400-6. doi: 10.1016/j.tibs.2007.08.001. Epub 2007 Aug 10. Trends Biochem Sci. 2007. PMID: 17693089 Review.
Cited by
-
DYT-THAP1: exploring gene expression in fibroblasts for potential biomarker discovery.Neurogenetics. 2024 Apr;25(2):141-147. doi: 10.1007/s10048-024-00752-0. Epub 2024 Mar 18. Neurogenetics. 2024. PMID: 38498291
-
Whole-Genome Scanning for Selection Signatures Reveals Candidate Genes Associated with Growth and Tail Length in Sheep.Animals (Basel). 2024 Feb 22;14(5):687. doi: 10.3390/ani14050687. Animals (Basel). 2024. PMID: 38473071 Free PMC article.
-
Bi-allelic truncating variants in CASP2 underlie a neurodevelopmental disorder with lissencephaly.Eur J Hum Genet. 2024 Jan;32(1):52-60. doi: 10.1038/s41431-023-01461-2. Epub 2023 Oct 26. Eur J Hum Genet. 2024. PMID: 37880421 Free PMC article.
-
Congenital hydrocephalus: new Mendelian mutations and evidence for oligogenic inheritance.Hum Genomics. 2023 Mar 2;17(1):16. doi: 10.1186/s40246-023-00464-w. Hum Genomics. 2023. PMID: 36859317 Free PMC article.
-
The Finnish genetic heritage in 2022 - from diagnosis to translational research.Dis Model Mech. 2022 Oct 1;15(10):dmm049490. doi: 10.1242/dmm.049490. Epub 2022 Oct 26. Dis Model Mech. 2022. PMID: 36285626 Free PMC article. Review.
References
-
- Bystron I., Blakemore C., Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat. Rev. Neurosci. 2008;9:110–122. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

