Metformin improves defective hematopoiesis and delays tumor formation in Fanconi anemia mice
- PMID: 27756748
- PMCID: PMC5159699
- DOI: 10.1182/blood-2015-11-683490
Metformin improves defective hematopoiesis and delays tumor formation in Fanconi anemia mice
Abstract
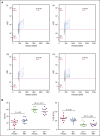
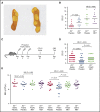
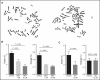
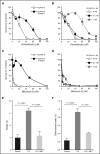
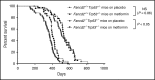
Fanconi anemia (FA) is an inherited bone marrow failure disorder associated with a high incidence of leukemia and solid tumors. Bone marrow transplantation is currently the only curative therapy for the hematopoietic complications of this disorder. However, long-term morbidity and mortality remain very high, and new therapeutics are badly needed. Here we show that the widely used diabetes drug metformin improves hematopoiesis and delays tumor formation in Fancd2-/- mice. Metformin is the first compound reported to improve both of these FA phenotypes. Importantly, the beneficial effects are specific to FA mice and are not seen in the wild-type controls. In this preclinical model of FA, metformin outperformed the current standard of care, oxymetholone, by improving peripheral blood counts in Fancd2-/- mice significantly faster. Metformin increased the size of the hematopoietic stem cell compartment and enhanced quiescence in hematopoietic stem and progenitor cells. In tumor-prone Fancd2-/-Trp53+/- mice, metformin delayed the onset of tumors and significantly extended the tumor-free survival time. In addition, we found that metformin and the structurally related compound aminoguanidine reduced DNA damage and ameliorated spontaneous chromosome breakage and radials in human FA patient-derived cells. Our results also indicate that aldehyde detoxification might be one of the mechanisms by which metformin reduces DNA damage in FA cells.
© 2016 by The American Society of Hematology.
Figures

Comment in
-
Metformin: treating the cause of Fanconi anemia?Blood. 2016 Dec 15;128(24):2748-2750. doi: 10.1182/blood-2016-11-748129. Blood. 2016. PMID: 27979864 Free PMC article.
Similar articles
-
Long-term combination therapy with metformin and oxymetholone in a Fanconi anemia mouse model.Pediatr Blood Cancer. 2024 Aug;71(8):e31030. doi: 10.1002/pbc.31030. Epub 2024 May 10. Pediatr Blood Cancer. 2024. PMID: 38733122
-
Radiologic differences between bone marrow stromal and hematopoietic progenitor cell lines from Fanconi Anemia (Fancd2(-/-)) mice.Radiat Res. 2014 Jan;181(1):76-89. doi: 10.1667/RR13405.1. Epub 2014 Jan 7. Radiat Res. 2014. PMID: 24397476 Free PMC article.
-
Oxymetholone therapy of fanconi anemia suppresses osteopontin transcription and induces hematopoietic stem cell cycling.Stem Cell Reports. 2015 Jan 13;4(1):90-102. doi: 10.1016/j.stemcr.2014.10.014. Epub 2014 Nov 26. Stem Cell Reports. 2015. PMID: 25434823 Free PMC article.
-
Current knowledge on the pathophysiology of Fanconi anemia: from genes to phenotypes.Int J Hematol. 2001 Jul;74(1):33-41. doi: 10.1007/BF02982547. Int J Hematol. 2001. PMID: 11530803 Review.
-
Beyond interstrand crosslinks repair: contribution of FANCD2 and other Fanconi Anemia proteins to the replication of DNA.Mutat Res. 2018 Mar;808:83-92. doi: 10.1016/j.mrfmmm.2017.09.004. Epub 2017 Sep 14. Mutat Res. 2018. PMID: 29031493 Review.
Cited by
-
Head and Neck Cancer Susceptibility and Metabolism in Fanconi Anemia.Cancers (Basel). 2022 Apr 18;14(8):2040. doi: 10.3390/cancers14082040. Cancers (Basel). 2022. PMID: 35454946 Free PMC article. Review.
-
Mitotic Errors Promote Genomic Instability and Leukemia in a Novel Mouse Model of Fanconi Anemia.Front Oncol. 2021 Nov 5;11:752933. doi: 10.3389/fonc.2021.752933. eCollection 2021. Front Oncol. 2021. PMID: 34804941 Free PMC article.
-
Importance of finding the bona fide target of the Fanconi anemia pathway.Genes Environ. 2019 Mar 6;41:6. doi: 10.1186/s41021-019-0122-y. eCollection 2019. Genes Environ. 2019. PMID: 30873250 Free PMC article. Review.
-
Phenformin increases early hematopoietic progenitors in the Jak2V617F murine model.Invest New Drugs. 2022 Jun;40(3):576-585. doi: 10.1007/s10637-022-01212-y. Epub 2022 Jan 11. Invest New Drugs. 2022. PMID: 35015172
-
Metformin Use and Severe Dengue in Diabetic Adults.Sci Rep. 2018 Feb 20;8(1):3344. doi: 10.1038/s41598-018-21612-6. Sci Rep. 2018. PMID: 29463812 Free PMC article.
References
-
- Kutler DI, Singh B, Satagopan J, et al. . A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood. 2003;101(4):1249-1256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

