RKIP and TBK1 form a positive feedback loop to promote type I interferon production in innate immunity
- PMID: 27753621
- PMCID: PMC5283588
- DOI: 10.15252/embj.201694060
RKIP and TBK1 form a positive feedback loop to promote type I interferon production in innate immunity
Abstract
TANK-binding kinase 1 (TBK1) activation is a central event in type I interferon production in anti-virus innate immunity. However, the regulatory mechanism underlying TBK1 activation remains unclear. Here we report that Raf kinase inhibitory protein (RKIP) is essential for TBK1 activation and type I interferon production triggered by viral infection. Upon viral infection, RKIP is phosphorylated at serine 109 (S109) by TBK1. Phosphorylation of RKIP enhances its interaction with TBK1 and in turn promotes TBK1 autophosphorylation. Mutation of RKIP S109 to alanine abrogates the interaction between RKIP and TBK1, and the anti-viral function of RKIP RKIP deficiency inhibits intracellular double-stranded RNA- or DNA-induced type I interferon production. Consistently, RKIP deficiency renders the mice more susceptible to vesicular stomatitis virus (VSV) and herpes simplex virus (HSV) infections. This study reveals a previously unrecognized positive feedback loop between RKIP and TBK1 that is essential for type I interferon production in anti-viral innate immunity.
Keywords: RKIP; TBK1; anti‐viral immunity; type I interferon.
© 2016 The Authors.
Figures

Gene array analysis of RKIP mRNA (GEO profile GDS5093) expression in peripheral blood samples from healthy donors and patients with dengue virus infection. Each symbol represents an individual subject; horizontal lines indicate the mean.
Real‐time PCR analysis of Ifnb1 mRNA in peritoneal macrophage isolated from RKIP +/+ or RKIP −/− mice infected with HSV (MOI 5) or Listeria monocytogenes (MOI 10).
cGAMP (100 nM) was delivered to BMDMs for the indicated times, and then, IFN‐β RNA and secreted protein were measured by real‐time PCR and ELISA, respectively.
ELISA of IL‐6, TNF‐α, and IFN‐β protein and real‐time PCR analysis of Il6, Tnf, and Ifnb1 mRNA in RAW264.7 cells treated with RKIP‐specific or scrambled siRNA, followed by infection with VSV (MOI 0.1).
ELISA of IL‐6, TNF‐α, and IFN‐β protein in RAW264.7 cells stably overexpressing RKIP, followed by treatment with VSV (MOI 0.1).
Real‐time PCR analysis of Tnf and Ifnb1 mRNA in RAW264.7 cells stably overexpressing RKIP and then followed by infection with HSV (MOI 5).
Immunoblot analysis of the expression of RKIP protein in primary macrophages with RKIP knockout or knockdown or RKIP stably overexpressing RAW264.7 transfectants.
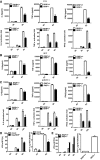
- A–C
Enzyme‐linked immunosorbent assay (ELISA) of IL‐6, TNF‐α, and IFN‐β protein and real‐time PCR analysis of Il6, Tnf, and IIfnb1 mRNA in peritoneal macrophages isolated from wild‐type and RKIP‐deficient mice treated with VSV (MOI 0.1) (A) and SEV (MOI 10) (B) or transfected with poly(I:C) (1 μg/ml) (C).
- D
Fluorescence‐activated cell sorting (FACS) and TCID50 (50% tissue culture infective dose) assessing the proliferation level of VSV. RAW264.7 cells line stably overexpressing flag‐RKIP and infected with VSV‐eGFP at MOI of 1 for the indicated time. The fusion protein fluorophore was analyzed by FACS. Then, fresh medium with the VSV‐eGFP infection was serially diluted on the monolayer of HEK293 cells for 3–7 days and TCID50 was measured.
- E
FACS analysis of wild‐type and RKIP‐deficient peritoneal macrophages infected with VSV‐eGFP at MOI of 1. The fresh medium with the VSV‐eGFP infection was collected after 9 h for TCID50 assessing.
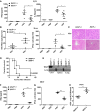
ELISA of IFN‐β and IL‐6 in serum obtained from wild‐type (RKIP +/+) and RKIP −/− mice 12 h after intraperitoneal injection with VSV (1 × 107 pfu/g) (n = 5 per group).
Real‐time PCR analysis of VSV expression and Ifnb1 mRNA in the liver from wild‐type and RKIP‐deficient mice treated as in (A).
Hematoxylin and eosin staining of liver sections from mice in (A). Scale bar, 100 μm.
Survival of ˜8‐week‐old RKIP +/+ and RKIP −/− mice given intravenous tail injection of VSV (1 × 108 pfu/g, i.p.) (n = 7 per group; Wilcoxon test).
Immunoblot assay of RKIP expression in lung, liver, and spleen from wild‐type and RKIP‐deficient mice.
ELISA of IFN‐β in serum from RKIP +/+ and RKIP −/− mice 6 h after intravenous injection of HSV (1 × 106 pfu/mouse) (n = 5 per group).
Real‐time PCR analysis of Ifnb1 gene expression in peritoneal macrophages 24 h after intravenous injection of HSV (1 × 106 pfu/mouse) (n = 5 per group).
Viral titers in brains obtained from RKIP +/+ and RKIP −/− mice 3 days after intravenous injection of HSV (1 × 106 pfu/mouse) (n = 5 per group).
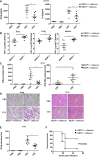
Wild‐type (RKIP +/+) and RKIP‐deficient (RKIP −/−) chimera mice were intraperitoneally injected with VSV (1 × 107 pfu/g); 12 h later ELISA of IFN‐β and IL‐6 in serum was performed (n = 6 per group).
Determination of VSV loads in organs by TCID50 assay 12 h after RKIP +/+ and RKIP −/− chimera mice (n = 6 per group) were intraperitoneally injected with VSV (1 × 107 pfu/g).
Real‐time PCR analysis of Ifnb1 mRNA and VSV expression in liver from RKIP +/+ and RKIP −/− chimera mice 12 h after intraperitoneal injection with VSV (1 × 107 pfu/g) (n = 6 per group).
Real‐time PCR analysis of Ifnb1 mRNA expression in peritoneal macrophages from RKIP +/+ and RKIP −/− chimera mice 12 h after intraperitoneal injection with VSV (1 × 107 pfu/g) (n = 6 per group).
Survival curve for 8‐week‐old wild‐type and RKIP‐deficient chimera mice challenged with VSV (1 × 108 pfu/g, i.v.) (n = 10 per group; Wilcoxon test).

Immunoblot analysis of p‐TBK1, p‐IKKi, p‐IRF3, p‐P38, p‐JNK1/2, p‐ERK1/2, and p‐IκBα in lysates of RKIP +/+ , RKIP +/−, and RKIP −/− peritoneal macrophages stimulated with VSV (MOI 0.1) for the indicated time. Actin was used as a loading control.
Immunoblot assay of RKIP +/+ , RKIP +/−, and RKIP −/− peritoneal macrophages treated with intracellular poly(I:C) (1 μg/ml). Actin was used as a loading control.
Immunoblot analysis of phosphorylated p‐TBK1, p‐IKKi, p‐IRF3, p‐P38, p‐JNK1/2, and p‐ERK1/2 in peritoneal macrophages transfected with RKIP‐specific or scrambled siRNA, followed by infection with VSV (MOI 0.1). Actin was used as a loading control.
Immunoblot analysis of p‐TBK1, p‐IKKi, p‐IRF3, p‐P38, p‐JNK1/2, p‐ERK1/2, and p‐IκBα in lysates of VSV‐treated RAW264.7 cells stably overexpressing RKIP. Actin was used as a loading control.
Immunoblot analysis in lysates of RAW264.7 cells stably overexpressing RKIP and treated with intracellular poly(I:C) (1 μg/ml). Actin was used as a loading control.
Luciferase activity in 293T cells transfected with plasmid encoding a luciferase reporter for IFN‐β (IFN‐β‐luc) or ISRE (ISRE‐luc), and an expression vector for RKIP (0, 50, 100, 200 ng each), together with plasmids encoding RIG‐I CARD, TBK1, IKKi, and IRF3 5D.
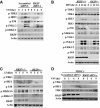
- A
Immunoblot analysis of p‐TBK1, p‐IRF3, p‐P38, p‐JNK1/2, p‐ERK1/2 in RAW264.7 cells stably transfected with RKIP‐specific or scrambled shRNA, followed by infection with VSV (MOI 0.1).
- B, C
Immunoblot analysis of p‐TBK1, p‐IRF3, p‐P38, p‐MKK3/6, p‐JNK1/2, p‐ERK1/2, p‐P65, and p‐TAK1 in lysates of wild‐type and RKIP‐deficient peritoneal macrophages stimulated with HSV (MOI 5) (B) or Listeria monocytogenes (MOI 10) (C).
- D
Immunoblot analysis of p‐TBK1 and p‐IRF3 in PBMCs treated with scrambled or RKIP‐specific siRNA for 48 h followed by stimulation with VSV (MOI 0.1) for the indicated times.
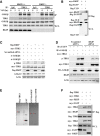
Coimmunoprecipitation analysis with anti‐TBK1 antibody in RKIP +/+ and RKIP −/− peritoneal macrophages infected with VSV (MOI 0.1), followed by immunoblotting with the indicated antibody.
Co‐immunoprecipitation and immunoblot analysis of 293T cells transfected with flag‐ULD alone or with myc‐RKIP. ULD, ubiquitin‐like domain.
RKIP promoting TBK1 phosphorylation is GSK3β independent. Immunoblot of extracts of 293T cells transfected with plasmid for myc‐TBK1, flag‐RKIP, together with GSK3β‐specific or scrambled siRNA.
GSK3β promotes TBK1 activation through a RKIP‐dependent mechanism. Immunoblot of extracts of 293T cells transfected with plasmid for myc‐TBK1, HA‐GSK3β, together with RKIP‐specific or scrambled siRNA.
Purified flag‐TBK1, GST, GST‐his‐RKIP, or GST‐his‐S109D proteins were detected with Coomassie brilliant blue. * indicates targeted band.
RKIP does not affect TBK1 self‐association. Coimmunoprecipitation and immunoblot analysis of 293T cells transfected with indicated combination of plasmid for flag‐TBK1, myc‐TBK1, myc‐RKIP, followed by immunoblot analysis with anti‐myc and anti‐flag antibodies.

Immunoblot of extracts of RAW264.7 cells stably overexpressing flag‐RKIP and infected with VSV (MOI 0.1) for the indicated times followed by immunoprecipitation (IP). WCL, whole‐cell lysates.
Coimmunoprecipitation and immunoblot analysis of 293T cells transfected with vector for myc‐RKIP alone or with the deletion mutants of TBK1. Numbers in parentheses indicate amino acids included in construct. FL, full length; KD, kinase domain; ULD, ubiquitin‐like domain; SDD, α‐helical scaffold domain; NBD, C‐terminal domain. * indicates targeted band.
Immunoblot of 293T cells transfected with plasmid for myc‐TBK1 together with plasmid for HA‐GSK3β or HA‐RKIP, followed by immunoprecipitation. * indicates targeted band.
In vitro kinase assay was carried out in the reaction mixture containing 2 mM ATP, 100 μM GST‐RKIP or 100 μM GST‐GFP, and 5 μM flag‐TBK1 at 30°C for 30 min and then followed by immunoblotting with the indicated antibody.
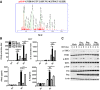
Tandem MS of phosphorylation sites S109 of RKIP.
ELISA of IL‐6 and IFN‐β, and real‐time PCR analysis of Il6 and Ifnb1 mRNA in RAW264.7 cells stably overexpressing flag‐RKIP, flag‐S109A, or flag‐S109D, followed by infection with HSV (MOI 5).
Immunoblot analysis of p‐TBK1, p‐IRF3, p‐P38 of cells in (B).

Real‐time PCR analysis of Il6, Tnf, and Ifnb1 mRNA in RAW264.7 cells stably overexpressing flag‐RKIP, flag‐S109A, and flag‐S109D, followed by infection with VSV (MOI 0.1).
Immunoblot analysis of p‐TBK1, p‐IRF3, and p‐P38 of cells as in (A).
FACS analysis of RAW264.7 cells stably overexpressing flag‐RKIP, flag‐S109A, and flag‐S109D infected with VSV‐eGFP at MOI of 1.
Immunoblot analysis of phosphorylated TBK1, IRF3, and P38 in lysates of RKIP +/+ or RKIP −/− bone marrow‐derived macrophages (BMDM) transfected with flag‐RKIP, flag‐S109A, and flag‐S109D, and then stimulated with VSV (MOI 0.1).
ELISA of IFN‐β and TNF‐α of cells in (D).

BMDM cells were infected with VSV (MOI 0.1) or HSV (MOI 5) for the indicated times. Cell lysates were analyzed by immunoblotting with the indicated antibodies.
BMDM cells were transfected with control or TBK1 siRNA. 72 h after transfection, cells were analyzed by immunoblotting with antibodies for phospho‐RKIP or TBK1.
Immunoblot analysis of RKIP phosphorylation in control or TBK1 −/− mouse embryonic fibroblasts after VSV (MOI 0.1) infection for the indicated times.
Recombinant flag‐tagged TBK1 was immunoprecipitated with anti‐flag M2 beads in flag‐TBK1‐transfected 293T cells. Followed this, in vitro kinase assay was carried out in the reaction mixture containing 2 mM ATP, 100 μM GST‐RKIP, or 100 μM GST‐GFP together with 5 μM flag‐TBK1 at 30°C for 30 min. Assay mixtures were immunoblotted with the indicated antibodies for phospho‐RKIP, flag, GST, or his.
Immunoblot analysis of extracts of RAW264.7 cells stably overexpressing flag‐RKIP, flag‐S109A, and flag‐S109D, and infected with VSV (MOI 0.1) for the indicated times, followed by immunoprecipitation with anti‐flag beads.
BMDM cells were infected with VSV (MOI 0.1) for the indicated times. The cell lysates were immunoprecipitated with anti‐TBK1 (left) or anti‐RKIP (right). The immunoprecipitates were analyzed by immunoblot with anti‐RKIP or anti‐TBK1 antibody. The levels of the endogenous RKIP, TBK1, and p‐RKIP were detected by immunoblot analysis.
Co‐immunoprecipitation and immunoblot analysis of 293T cells transfected with myc‐TBK1 alone or with flag‐RKIP, flag‐S109A, or flag‐S109D.
GST pull‐down experiment was performed with 1 μg fusion protein GST‐GFP, GST‐his‐RKIP, or GST‐his‐S109D mixed with 20 μl pre‐cleared agarose beads in 800 μl reaction medium, followed by adding 1 μg flag‐TBK1 and incubating at 4°C for 3 h with gentle rotation. Pull‐down (lane 1–3) and input samples (lane 4) were separated by SDS–PAGE followed by immunoblotting with anti‐GST or anti‐flag antibody.
Immunoprecipitation and immunoblot analysis of 293T cells transfected with indicated combinations of vector for myc‐TBK1, flag‐RKIP, flag‐S109A, flag‐S109D, HA‐GSK3β or flag‐GFP.
In vitro assay was carried out in the reaction mixture containing 2 mM ATP, 50 μM GST‐TBK1 together with 100 μM GST‐his‐RKIP, 100 μM GST‐his‐S109D, 100 μM GST‐his‐S109A, or 100 μM GST, respectively, at 30°C for 30 min, followed by immunoblotting with anti‐p‐TBK1 or anti‐GST antibody.
Similar articles
-
Phosphatase PP4 Negatively Regulates Type I IFN Production and Antiviral Innate Immunity by Dephosphorylating and Deactivating TBK1.J Immunol. 2015 Oct 15;195(8):3849-57. doi: 10.4049/jimmunol.1403083. Epub 2015 Sep 11. J Immunol. 2015. PMID: 26363053
-
Raf Kinase Inhibitor Protein Preferentially Promotes TLR3-Triggered Signaling and Inflammation.J Immunol. 2017 May 15;198(10):4086-4095. doi: 10.4049/jimmunol.1601672. Epub 2017 Apr 14. J Immunol. 2017. PMID: 28411188
-
E3 ubiquitin ligase RNF128 promotes innate antiviral immunity through K63-linked ubiquitination of TBK1.Nat Immunol. 2016 Dec;17(12):1342-1351. doi: 10.1038/ni.3588. Epub 2016 Oct 24. Nat Immunol. 2016. PMID: 27776110
-
TANK-binding kinase 1 as a novel therapeutic target for viral diseases.Expert Opin Ther Targets. 2019 May;23(5):437-446. doi: 10.1080/14728222.2019.1601702. Epub 2019 Apr 10. Expert Opin Ther Targets. 2019. PMID: 30932713 Review.
-
[Research progress on the role of TANK-binding kinase 1 in anti-virus innate immune response].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2016 May 25;45(5):550-557. doi: 10.3785/j.issn.1008-9292.2016.09.16. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2016. PMID: 28087918 Free PMC article. Review. Chinese.
Cited by
-
AAV induces hepatic necroptosis and carcinoma in diabetic and obese mice dependent on Pebp1 pathway.EMBO Mol Med. 2023 Jul 10;15(7):e17230. doi: 10.15252/emmm.202217230. Epub 2023 Jun 5. EMBO Mol Med. 2023. PMID: 37272212 Free PMC article.
-
Changes in Expression of Tumor Suppressor Gene RKIP Impact How Cancers Interact with Their Complex Environment.Cancers (Basel). 2023 Feb 2;15(3):958. doi: 10.3390/cancers15030958. Cancers (Basel). 2023. PMID: 36765912 Free PMC article. Review.
-
RKIP suppresses the influenza A virus‑induced airway inflammatory response via the ERK/MAPK pathway.Int J Mol Med. 2023 Jan;51(1):1. doi: 10.3892/ijmm.2022.5204. Epub 2022 Nov 16. Int J Mol Med. 2023. PMID: 36382638 Free PMC article.
-
A Negative Regulatory Role for RKIP in Breast Cancer Immune Response.Cancers (Basel). 2022 Jul 24;14(15):3605. doi: 10.3390/cancers14153605. Cancers (Basel). 2022. PMID: 35892864 Free PMC article.
-
PEBP balances apoptosis and autophagy in whitefly upon arbovirus infection.Nat Commun. 2022 Feb 11;13(1):846. doi: 10.1038/s41467-022-28500-8. Nat Commun. 2022. PMID: 35149691 Free PMC article.
References
-
- Al‐Mulla F, Bitar MS, Al‐Maghrebi M, Behbehani AI, Al‐Ali W, Rath O, Doyle B, Tan KY, Pitt A, Kolch W (2011) Raf kinase inhibitor protein RKIP enhances signaling by glycogen synthase kinase‐3beta. Cancer Res 71: 1334–1343 - PubMed
-
- Al‐Mulla F, Bitar MS, Taqi Z, Yeung K (2013) RKIP: much more than Raf kinase inhibitory protein. J Cell Physiol 228: 1688–1702 - PubMed
-
- Ausubel FM (2005) Are innate immune signaling pathways in plants and animals conserved? Nat Immunol 6: 973–979 - PubMed
-
- Barbalat R, Ewald SE, Mouchess ML, Barton GM (2011) Nucleic acid recognition by the innate immune system. Annu Rev Immunol 29: 185–214 - PubMed
-
- Bernier I, Jolles P (1984) Purification and characterization of a basic 23 kDa cytosolic protein from bovine brain. Biochim Biophys Acta 790: 174–181 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

