A tissue checkpoint regulates type 2 immunity
- PMID: 27749840
- PMCID: PMC5275767
- DOI: 10.1038/ni.3582
A tissue checkpoint regulates type 2 immunity
Abstract
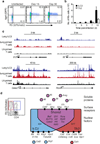
Group 2 innate lymphoid cells (ILC2s) and CD4+ type 2 helper T cells (TH2 cells) are defined by their similar effector cytokines, which together mediate the features of allergic immunity. We found that tissue ILC2s and TH2 cells differentiated independently but shared overlapping effector function programs that were mediated by exposure to the tissue-derived cytokines interleukin 25 (IL-25), IL-33 and thymic stromal lymphopoietin (TSLP). Loss of these three tissue signals did not affect lymph node priming, but abrogated the terminal differentiation of effector TH2 cells and adaptive lung inflammation in a T cell-intrinsic manner. Our findings suggest a mechanism by which diverse perturbations can activate type 2 immunity and reveal a shared local-tissue-elicited checkpoint that can be exploited to control both innate and adaptive allergic inflammation.
Figures


Comment in
-
T cells: A tissue checkpoint for TH2s.Nat Rev Immunol. 2016 Nov 25;16(12):717. doi: 10.1038/nri.2016.130. Nat Rev Immunol. 2016. PMID: 27885277 No abstract available.
Similar articles
-
Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses.Clin Exp Allergy. 2009 Jun;39(6):798-806. doi: 10.1111/j.1365-2222.2009.03241.x. Epub 2009 Apr 7. Clin Exp Allergy. 2009. PMID: 19400908 Free PMC article. Review.
-
Combinatorial targeting of TSLP, IL-25, and IL-33 in type 2 cytokine-driven inflammation and fibrosis.Sci Transl Med. 2016 May 4;8(337):337ra65. doi: 10.1126/scitranslmed.aaf1938. Sci Transl Med. 2016. PMID: 27147589
-
Thymic stromal lymphopoietin signaling in CD4(+) T cells is required for TH2 memory.J Allergy Clin Immunol. 2015 Mar;135(3):781-91.e3. doi: 10.1016/j.jaci.2014.09.015. Epub 2014 Oct 24. J Allergy Clin Immunol. 2015. PMID: 25441291 Free PMC article.
-
Innate and adaptive type 2 immunity in lung allergic inflammation.Immunol Rev. 2017 Jul;278(1):162-172. doi: 10.1111/imr.12557. Immunol Rev. 2017. PMID: 28658559 Review.
-
Allergen-Experienced Group 2 Innate Lymphoid Cells Acquire Memory-like Properties and Enhance Allergic Lung Inflammation.Immunity. 2016 Jul 19;45(1):198-208. doi: 10.1016/j.immuni.2016.06.017. Epub 2016 Jul 12. Immunity. 2016. PMID: 27421705
Cited by
-
IL-25 Orchestrates Activation of Th Cells via Conventional Dendritic Cells in Tissue to Exacerbate Chronic House Dust Mite-Induced Asthma Pathology.J Immunol. 2019 Oct 15;203(8):2319-2327. doi: 10.4049/jimmunol.1900254. Epub 2019 Sep 11. J Immunol. 2019. PMID: 31511356 Free PMC article.
-
Finding a Niche: Tissue Immunity and Innate Lymphoid Cells.Adv Exp Med Biol. 2022;1365:57-73. doi: 10.1007/978-981-16-8387-9_5. Adv Exp Med Biol. 2022. PMID: 35567741
-
Tuft cell integration of luminal states and interaction modules in tissues.Pflugers Arch. 2021 Nov;473(11):1713-1722. doi: 10.1007/s00424-021-02630-2. Epub 2021 Oct 11. Pflugers Arch. 2021. PMID: 34635955 Free PMC article. Review.
-
Fungal-mediated lung allergic airway disease: The critical role of macrophages and dendritic cells.PLoS Pathog. 2022 Jul 14;18(7):e1010608. doi: 10.1371/journal.ppat.1010608. eCollection 2022 Jul. PLoS Pathog. 2022. PMID: 35834490 Free PMC article. Review.
-
Secreted Phospholipase A2 Group X Acts as an Adjuvant for Type 2 Inflammation, Leading to an Allergen-Specific Immune Response in the Lung.J Immunol. 2020 Jun 15;204(12):3097-3107. doi: 10.4049/jimmunol.2000102. Epub 2020 Apr 27. J Immunol. 2020. PMID: 32341057 Free PMC article.
References
METHODS-ONLY REFERENCES
-
- Picard Tools. 2015 < http://broadinstitute.github.io/picard/>.
MeSH terms
Substances
Grants and funding
- T32 AI007334/AI/NIAID NIH HHS/United States
- T32 GM067547/GM/NIGMS NIH HHS/United States
- T32 GM007618/GM/NIGMS NIH HHS/United States
- P01 HL107202/HL/NHLBI NIH HHS/United States
- DP5 OD012178/OD/NIH HHS/United States
- K08 AI113143/AI/NIAID NIH HHS/United States
- R37 AI026918/AI/NIAID NIH HHS/United States
- K08 DK101604/DK/NIDDK NIH HHS/United States
- R01 AI026918/AI/NIAID NIH HHS/United States
- R01 HL128903/HL/NHLBI NIH HHS/United States
- R01 AI030663/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

