Potent and Selective Peptide-based Inhibition of the G Protein Gαq
- PMID: 27742837
- PMCID: PMC5207258
- DOI: 10.1074/jbc.M116.740407
Potent and Selective Peptide-based Inhibition of the G Protein Gαq
Abstract
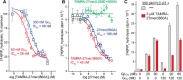
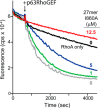
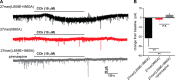
In contrast to G protein-coupled receptors, for which chemical and peptidic inhibitors have been extensively explored, few compounds are available that directly modulate heterotrimeric G proteins. Active Gαq binds its two major classes of effectors, the phospholipase C (PLC)-β isozymes and Rho guanine nucleotide exchange factors (RhoGEFs) related to Trio, in a strikingly similar fashion: a continuous helix-turn-helix of the effectors engages Gαq within its canonical binding site consisting of a groove formed between switch II and helix α3. This information was exploited to synthesize peptides that bound active Gαq in vitro with affinities similar to full-length effectors and directly competed with effectors for engagement of Gαq A representative peptide was specific for active Gαq because it did not bind inactive Gαq or other classes of active Gα subunits and did not inhibit the activation of PLC-β3 by Gβ1γ2 In contrast, the peptide robustly prevented activation of PLC-β3 or p63RhoGEF by Gαq; it also prevented G protein-coupled receptor-promoted neuronal depolarization downstream of Gαq in the mouse prefrontal cortex. Moreover, a genetically encoded form of this peptide flanked by fluorescent proteins inhibited Gαq-dependent activation of PLC-β3 at least as effectively as a dominant-negative form of full-length PLC-β3. These attributes suggest that related, cell-penetrating peptides should effectively inhibit active Gαq in cells and that these and genetically encoded sequences may find application as molecular probes, drug leads, and biosensors to monitor the spatiotemporal activation of Gαq in cells.
Keywords: G protein; inhibitor; p63; peptide interaction; phospholipase C.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
Designer proteins that competitively inhibit Gαq by targeting its effector site.J Biol Chem. 2021 Dec;297(6):101348. doi: 10.1016/j.jbc.2021.101348. Epub 2021 Oct 27. J Biol Chem. 2021. PMID: 34715131 Free PMC article.
-
The binding of activated Gαq to phospholipase C-β exhibits anomalous affinity.J Biol Chem. 2017 Oct 6;292(40):16787-16801. doi: 10.1074/jbc.M117.809673. Epub 2017 Aug 24. J Biol Chem. 2017. PMID: 28842497 Free PMC article.
-
Gβγ signaling to the chemotactic effector P-REX1 and mammalian cell migration is directly regulated by Gαq and Gα13 proteins.J Biol Chem. 2019 Jan 11;294(2):531-546. doi: 10.1074/jbc.RA118.006254. Epub 2018 Nov 16. J Biol Chem. 2019. PMID: 30446620 Free PMC article.
-
Decoding Gαq signaling.Life Sci. 2016 May 1;152:99-106. doi: 10.1016/j.lfs.2016.03.037. Epub 2016 Mar 22. Life Sci. 2016. PMID: 27012764 Review.
-
Gαq signalling: the new and the old.Cell Signal. 2014 May;26(5):833-48. doi: 10.1016/j.cellsig.2014.01.010. Epub 2014 Jan 17. Cell Signal. 2014. PMID: 24440667 Review.
Cited by
-
Expression of the C-Terminal Domain of Phospholipase Cβ3 Inhibits Signaling via Gαq-Coupled Receptors and Transient Receptor Potential Channels.Int J Mol Sci. 2022 Aug 24;23(17):9590. doi: 10.3390/ijms23179590. Int J Mol Sci. 2022. PMID: 36076982 Free PMC article.
-
Dissociation of the G protein βγ from the Gq-PLCβ complex partially attenuates PIP2 hydrolysis.J Biol Chem. 2021 Jan-Jun;296:100702. doi: 10.1016/j.jbc.2021.100702. Epub 2021 Apr 24. J Biol Chem. 2021. PMID: 33901492 Free PMC article.
-
Uveal melanoma: progress in molecular biology and therapeutics.Ther Adv Med Oncol. 2020 Oct 22;12:1758835920965852. doi: 10.1177/1758835920965852. eCollection 2020. Ther Adv Med Oncol. 2020. PMID: 33149769 Free PMC article. Review.
-
Constitutive, Basal, and β-Alanine-Mediated Activation of the Human Mas-Related G Protein-Coupled Receptor D Induces Release of the Inflammatory Cytokine IL-6 and Is Dependent on NF-κB Signaling.Int J Mol Sci. 2021 Dec 9;22(24):13254. doi: 10.3390/ijms222413254. Int J Mol Sci. 2021. PMID: 34948051 Free PMC article.
-
Tetrahydroimidazo[1,2-a]pyrazine Derivatives: Synthesis and Evaluation as Gαq -Protein Ligands.Chemistry. 2020 Oct 1;26(55):12615-12623. doi: 10.1002/chem.202001446. Epub 2020 Sep 7. Chemistry. 2020. PMID: 32428383 Free PMC article.
References
-
- Wettschureck N., and Offermanns S. (2005) Mammalian G proteins and their cell type specific functions. Physiol. Rev. 85, 1159–1204 - PubMed
-
- Rozengurt E. (2007) Mitogenic signaling pathways induced by G protein-coupled receptors. J. Cell. Physiol. 213, 589–602 - PubMed
-
- Gainetdinov R. R., Premont R. T., Bohn L. M., Lefkowitz R. J., and Caron M. G. (2004) Desensitization of G protein-coupled receptors and neuronal functions. Annu. Rev. Neurosci. 27, 107–144 - PubMed
-
- Hubbard K. B., and Hepler J. R. (2006) Cell signalling diversity of the Gαq family of heterotrimeric G proteins. Cell Signal. 18, 135–150 - PubMed
-
- Wall M. A., Coleman D. E., Lee E., Iñiguez-Lluhi J. A., Posner B. A., Gilman A. G., and Sprang S. R. (1995) The structure of the G protein heterotrimer Gi(α1β1γ2). Cell 83, 1047–1058 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

