The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype
- PMID: 27737891
- PMCID: PMC5179338
- DOI: 10.1182/blood-2016-07-730556
The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype
Abstract
Despite genetic heterogeneity, myelodysplastic syndromes (MDSs) share features of cytological dysplasia and ineffective hematopoiesis. We report that a hallmark of MDSs is activation of the NLRP3 inflammasome, which drives clonal expansion and pyroptotic cell death. Independent of genotype, MDS hematopoietic stem and progenitor cells (HSPCs) overexpress inflammasome proteins and manifest activated NLRP3 complexes that direct activation of caspase-1, generation of interleukin-1β (IL-1β) and IL-18, and pyroptotic cell death. Mechanistically, pyroptosis is triggered by the alarmin S100A9 that is found in excess in MDS HSPCs and bone marrow plasma. Further, like somatic gene mutations, S100A9-induced signaling activates NADPH oxidase (NOX), increasing levels of reactive oxygen species (ROS) that initiate cation influx, cell swelling, and β-catenin activation. Notably, knockdown of NLRP3 or caspase-1, neutralization of S100A9, and pharmacologic inhibition of NLRP3 or NOX suppress pyroptosis, ROS generation, and nuclear β-catenin in MDSs and are sufficient to restore effective hematopoiesis. Thus, alarmins and founder gene mutations in MDSs license a common redox-sensitive inflammasome circuit, which suggests new avenues for therapeutic intervention.
© 2016 by The American Society of Hematology.
Figures
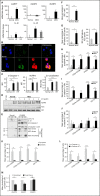
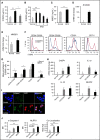

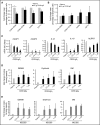
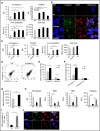

Comment in
-
Dying a fiery death: pyroptosis in MDS.Blood. 2016 Dec 22;128(25):2875-2877. doi: 10.1182/blood-2016-10-746719. Blood. 2016. PMID: 28007731 No abstract available.
Similar articles
-
The central role of inflammatory signaling in the pathogenesis of myelodysplastic syndromes.Blood. 2019 Mar 7;133(10):1039-1048. doi: 10.1182/blood-2018-10-844654. Epub 2019 Jan 22. Blood. 2019. PMID: 30670444 Free PMC article. Review.
-
Cellular senescence induced by S100A9 in mesenchymal stromal cells through NLRP3 inflammasome activation.Aging (Albany NY). 2019 Nov 14;11(21):9626-9642. doi: 10.18632/aging.102409. Epub 2019 Nov 14. Aging (Albany NY). 2019. PMID: 31727865 Free PMC article.
-
Unraveling the Pathogenesis of MDS: The NLRP3 Inflammasome and Pyroptosis Drive the MDS Phenotype.Front Oncol. 2016 Jun 16;6:151. doi: 10.3389/fonc.2016.00151. eCollection 2016. Front Oncol. 2016. PMID: 27379212 Free PMC article. Review.
-
Oxidized Mitochondrial DNA Engages TLR9 to Activate the NLRP3 Inflammasome in Myelodysplastic Syndromes.Int J Mol Sci. 2023 Feb 15;24(4):3896. doi: 10.3390/ijms24043896. Int J Mol Sci. 2023. PMID: 36835307 Free PMC article.
-
Somatic gene mutations expose cytoplasmic DNA to co-opt the cGAS/STING/NLRP3 axis in myelodysplastic syndromes.JCI Insight. 2022 Aug 8;7(15):e159430. doi: 10.1172/jci.insight.159430. JCI Insight. 2022. PMID: 35788117 Free PMC article.
Cited by
-
The inflammasomes: crosstalk between innate immunity and hematology.Inflamm Res. 2022 Dec;71(12):1403-1416. doi: 10.1007/s00011-022-01646-3. Epub 2022 Oct 20. Inflamm Res. 2022. PMID: 36266587 Review.
-
Biological drivers of clinical phenotype in myelofibrosis.Leukemia. 2023 Feb;37(2):255-264. doi: 10.1038/s41375-022-01767-y. Epub 2022 Nov 24. Leukemia. 2023. PMID: 36434065 Free PMC article. Review.
-
Navigating the contested borders between myelodysplastic syndrome and acute myeloid leukemia.Front Oncol. 2022 Oct 28;12:1033534. doi: 10.3389/fonc.2022.1033534. eCollection 2022. Front Oncol. 2022. PMID: 36387170 Free PMC article. Review.
-
Alarming and Calming: Opposing Roles of S100A8/S100A9 Dimers and Tetramers on Monocytes.Adv Sci (Weinh). 2022 Dec;9(36):e2201505. doi: 10.1002/advs.202201505. Epub 2022 Oct 30. Adv Sci (Weinh). 2022. PMID: 36310133 Free PMC article.
-
S100a9 deficiency accelerates MDS-associated tumor escape via PD-1/PD-L1 overexpression.Acta Biochim Biophys Sin (Shanghai). 2023 Feb 25;55(2):194-201. doi: 10.3724/abbs.2023015. Acta Biochim Biophys Sin (Shanghai). 2023. PMID: 36810783 Free PMC article.
References
-
- List A, Kurtin S, Roe DJ, et al. . Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352(6):549-557. - PubMed
-
- Span LF, Rutten E, Gemmink A, Boezeman JB, Raymakers RA, de Witte T. Bone marrow mononuclear cells of MDS patients are characterized by in vitro proliferation and increased apoptosis independently of stromal interactions. Leuk Res. 2007;31(12):1659-1667. - PubMed
-
- Garcia-Manero G. Myelodysplastic syndromes: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89(1):97-108. - PubMed
-
- Yoshida K, Sanada M, Shiraishi Y, et al. . Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478(7367):64-69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

