Sodium Butyrate Promotes Reassembly of Tight Junctions in Caco-2 Monolayers Involving Inhibition of MLCK/MLC2 Pathway and Phosphorylation of PKCβ2
- PMID: 27735862
- PMCID: PMC5085728
- DOI: 10.3390/ijms17101696
Sodium Butyrate Promotes Reassembly of Tight Junctions in Caco-2 Monolayers Involving Inhibition of MLCK/MLC2 Pathway and Phosphorylation of PKCβ2
Abstract
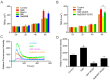
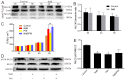
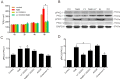
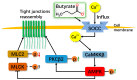
As a physiological small molecular product from the microbial fermentation of dietary fibers, butyrate plays an important role in maintaining intestinal health. Our previous works have proved that the effect of sodium butyrate (NaB) on the intestinal barrier function is mediated by activation of AMP-activated protein kinase (AMPK). However, the detailed pathway involved remains unknown. Using the calcium switch assay in the Caco-2 cell monolayer model, we found here that NaB activated AMPK mainly by increasing the calcium level, but not the ATP concentration, via promoting store-operated calcium entry (SOCE). Upon the activation of AMPK, NaB promoted the reassembly of tight junctions (TJs) based on reducing the phosphorylation of myosin II regulatory light chain (MLC2) at Ser19 and increasing phosphorylation of protein kinase C β2 (PKCβ2) at Ser660. Inhibiting (protein kinase C β) PKCβ blocked the reassembly of TJs induced by NaB in the barrier monolayer model. These results indicated that NaB could activate the calcium/calmodulin-dependent protein kinase kinase β (CaMKKβ) pathway to mediate AMPK phosphorylating, which then inhibited the phosphorylation of MLC2 and promoted the phosphorylation of PKCβ2, respectively, so that the downstream molecules of AMPK coordinately contributed to the reassembly of TJs in the Caco-2 barrier model. These results suggested a potential mechanism of butyrate for intestine homeostasis and protection.
Keywords: (protein kinase C β) PKCβ; Caco-2; butyrate; myosin II regulatory light chain (MLC2); myosin light chain kinase (MLCK); tight junction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Mycophenolic acid mediated disruption of the intestinal epithelial tight junctions.Exp Cell Res. 2014 Apr 1;322(2):277-89. doi: 10.1016/j.yexcr.2014.01.021. Epub 2014 Feb 7. Exp Cell Res. 2014. PMID: 24509232
-
Wogonoside alleviates colitis by improving intestinal epithelial barrier function via the MLCK/pMLC2 pathway.Phytomedicine. 2020 Mar;68:153179. doi: 10.1016/j.phymed.2020.153179. Epub 2020 Feb 3. Phytomedicine. 2020. PMID: 32062328
-
LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms.Gastroenterology. 2007 Jun;132(7):2383-94. doi: 10.1053/j.gastro.2007.02.052. Epub 2007 Feb 27. Gastroenterology. 2007. PMID: 17570213 Free PMC article.
-
AMPK in regulation of apical junctions and barrier function of intestinal epithelium.Tissue Barriers. 2018;6(2):1-13. doi: 10.1080/21688370.2018.1487249. Epub 2018 Aug 21. Tissue Barriers. 2018. PMID: 30130441 Free PMC article. Review.
-
Show me the pathway! Regulation of paracellular permeability by Na(+)-glucose cotransport.Adv Drug Deliv Rev. 2000 Jun 30;41(3):265-81. doi: 10.1016/s0169-409x(00)00046-6. Adv Drug Deliv Rev. 2000. PMID: 10854686 Review.
Cited by
-
Carbohydrate-to-Fiber Ratio, a Marker of Dietary Intake, as an Indicator of Depressive Symptoms.Cureus. 2021 Sep 15;13(9):e17996. doi: 10.7759/cureus.17996. eCollection 2021 Sep. Cureus. 2021. PMID: 34667672 Free PMC article.
-
The New Buffer Salt-Protected Sodium Butyrate Promotes Growth Performance by Improving Intestinal Histomorphology, Barrier Function, Antioxidative Capacity, and Microbiota Community of Broilers.Biology (Basel). 2024 May 1;13(5):317. doi: 10.3390/biology13050317. Biology (Basel). 2024. PMID: 38785799 Free PMC article.
-
Dietary copper improves intestinal structural integrity in juvenile grass carp (Ctenopharyngodon idella) probably related to its increased intestinal antioxidant capacity and apical junction complex.Anim Nutr. 2024 Apr 4;18:96-106. doi: 10.1016/j.aninu.2024.02.005. eCollection 2024 Sep. Anim Nutr. 2024. PMID: 39056059 Free PMC article.
-
Microbial-Derived Tryptophan Catabolites, Kidney Disease and Gut Inflammation.Toxins (Basel). 2022 Sep 18;14(9):645. doi: 10.3390/toxins14090645. Toxins (Basel). 2022. PMID: 36136583 Free PMC article. Review.
-
Mechanisms of Blood-Brain Barrier Protection by Microbiota-Derived Short-Chain Fatty Acids.Cells. 2023 Feb 18;12(4):657. doi: 10.3390/cells12040657. Cells. 2023. PMID: 36831324 Free PMC article. Review.
References
-
- Bergman E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990;70:567–590. - PubMed
-
- Gill R.K., Dudeja P.K. A novel facet to consider for the effects of butyrate on its target cells. Focus on “the short-chain fatty acid butyrate is a substrate of breast cancer resistance protein”. Am. J. Physiol. Cell Physiol. 2011;301:C977–C979. doi: 10.1152/ajpcell.00290.2011. - DOI - PMC - PubMed
-
- Harten S.K., Shukla D., Barod R., Hergovich A., Balda M.S., Matter K., Esteban M.A., Maxwell P.H. Regulation of renal epithelial tight junctions by the von Hippel-Lindau tumor suppressor gene involves occludin and claudin 1 and is independent of E-cadherin. Mol. Biol. Cell. 2009;20:1089–1101. doi: 10.1091/mbc.E08-06-0566. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

