Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials
- PMID: 27722750
- PMCID: PMC7505121
- DOI: 10.1001/jamaoncol.2016.3797
Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials
Erratum in
-
Incorrect Hazard Ratio in Figure 2.JAMA Oncol. 2017 Dec 1;3(12):1742. doi: 10.1001/jamaoncol.2017.4136. JAMA Oncol. 2017. PMID: 29075749 No abstract available.
Abstract
Importance: Metastatic colorectal cancer (mCRC) is heterogeneous, and primary tumors arising from different regions of the colon are clinically and molecularly distinct.
Objective: To examine the prognostic and predictive value of primary tumor location in patients with RAS wild-type (wt) mCRC treated with first-line fluorouracil, leucovorin, and irinotecan (FOLFIRI) plus cetuximab in the Cetuximab Combined With Irinotecan in First-line Therapy for Metastatic Colorectal Cancer (CRYSTAL) trial and FOLFIRI Plus Cetuximab Versus FOLFIRI Plus Bevacizumab as First-Line Treatment For Patients With Metastatic Colorectal Cancer (FIRE-3) trial.
Design, setting, and participants: In this retrospective analysis patients with RAS wt metastatic colorectal cancer from the CRYSTAL and FIRE-3 trials were classified as having left-sided or right-sided mCRC, defined, respectively, as patients whose tumors originated in the splenic flexure, descending colon, sigmoid colon, or rectum vs appendix, cecum, ascending colon, hepatic flexure, or transverse colon.
Main outcomes and measures: Progression-free survival (PFS), overall survival (OS), and objective response rate (ORR) were assessed according to tumor location and treatment arm.
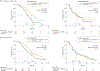
Results: In the RAS wt populations of the CRYSTAL and FIRE-3 trials, patients with left-sided tumors (n = 142 and n = 157, respectively) had markedly superior PFS, OS, and ORR compared with patients with right-sided tumors (n = 33 and n = 38, respectively). Among CRYSTAL and FIRE-3 study patients with RAS wt left-sided tumors, FOLFIRI plus cetuximab significantly improved OS relative to the respective comparators (FOLFIRI and FOLFIRI plus bevacizumab); in contrast, in RAS wt patients with poor-prognosis right-sided tumors, limited efficacy benefits were observed upon the addition of cetuximab to FOLFIRI in CRYSTAL, and comparable outcomes were observed between the FOLFIRI plus cetuximab and FOLFIRI plus bevacizumab arms of FIRE-3. A significant interaction was observed between primary tumor location and treatment for OS (CRYSTAL: hazard ratio [HR], 1.95; 95% CI, 1.09-3.48 and FIRE-3: HR, 0.40; 95% CI, 0.23-0.70) within the RAS wt populations of both studies in multivariable models that also included sex, prior adjuvant therapy, and BRAF mutational status.
Conclusions and relevance: In the RAS wt populations of CRYSTAL and FIRE-3, patients with left-sided tumors had a markedly better prognosis than those with right-sided tumors. First-line FOLFIRI plus cetuximab clearly benefitted patients with left-sided tumors (vs FOLFIRI or FOLFIRI plus bevacizumab, respectively), whereas patients with right-sided tumors derived limited benefit from standard treatments.
Trial registration: clinicaltrials.gov Identifiers: CRYSTAL, NCT00154102, and FIRE-3, NCT00433927.
Conflict of interest statement
Figures

Comment in
-
Primary tumor location found to impact prognosis and response to therapy in patients with metastatic colorectal cancer.CA Cancer J Clin. 2017 Jul 8;67(4):259-260. doi: 10.3322/caac.21372. Epub 2017 May 26. CA Cancer J Clin. 2017. PMID: 28548692 No abstract available.
Similar articles
-
Primary tumor location and survival in colorectal cancer: A retrospective cohort study.World J Gastrointest Oncol. 2020 Apr 15;12(4):405-423. doi: 10.4251/wjgo.v12.i4.405. World J Gastrointest Oncol. 2020. PMID: 32368319 Free PMC article.
-
Consensus molecular subgroups (CMS) of colorectal cancer (CRC) and first-line efficacy of FOLFIRI plus cetuximab or bevacizumab in the FIRE3 (AIO KRK-0306) trial.Ann Oncol. 2019 Nov 1;30(11):1796-1803. doi: 10.1093/annonc/mdz387. Ann Oncol. 2019. PMID: 31868905 Free PMC article. Clinical Trial.
-
Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials.Ann Oncol. 2017 Aug 1;28(8):1713-1729. doi: 10.1093/annonc/mdx175. Ann Oncol. 2017. PMID: 28407110 Free PMC article.
-
A meta-analysis of efficacy and safety data from head-to-head first-line trials of epidermal growth factor receptor inhibitors versus bevacizumab in adult patients with RAS wild-type metastatic colorectal cancer by sidedness.Eur J Cancer. 2024 May;202:113975. doi: 10.1016/j.ejca.2024.113975. Epub 2024 Mar 1. Eur J Cancer. 2024. PMID: 38442645 Review.
-
FOLFOX plus anti-epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) is an effective first-line treatment for patients with RAS-wild left-sided metastatic colorectal cancer: A meta-analysis.Medicine (Baltimore). 2018 Mar;97(10):e0097. doi: 10.1097/MD.0000000000010097. Medicine (Baltimore). 2018. PMID: 29517682 Free PMC article. Review.
Cited by
-
The prognostic factors of primary colorectal sarcoma and the clinical outcomes of negative lymph node dissection.Ann Transl Med. 2021 Feb;9(3):250. doi: 10.21037/atm-20-4286. Ann Transl Med. 2021. PMID: 33708877 Free PMC article.
-
Tumor location matters, next generation sequencing mutation profiling of left-sided, rectal, and right-sided colorectal tumors in 552 patients.Sci Rep. 2024 Feb 26;14(1):4619. doi: 10.1038/s41598-024-55139-w. Sci Rep. 2024. PMID: 38409377 Free PMC article.
-
The Role of Maintenance Strategies in Metastatic Colorectal Cancer: A Systematic Review and Network Meta-analysis of Randomized Clinical Trials.JAMA Oncol. 2020 Mar 1;6(3):e194489. doi: 10.1001/jamaoncol.2019.4489. Epub 2020 Mar 12. JAMA Oncol. 2020. PMID: 31855256 Free PMC article.
-
Tumor Sidedness and Enriched Gene Groups for Efficacy of First-line Cetuximab Treatment in Metastatic Colorectal Cancer.Mol Cancer Ther. 2018 Dec;17(12):2788-2795. doi: 10.1158/1535-7163.MCT-18-0694. Epub 2018 Oct 1. Mol Cancer Ther. 2018. PMID: 30275242 Free PMC article.
-
Another Chapter of the Right Versus Left Story: Is Primary Tumor Location a Prognostic Feature in RAS Mutant Metastatic Colorectal Cancer?Oncologist. 2019 Feb;24(2):e77-e79. doi: 10.1634/theoncologist.2018-0180. Epub 2018 Aug 17. Oncologist. 2019. PMID: 30120156 Free PMC article.
References
-
- Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–1417. - PubMed
-
- Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011–2019. - PubMed
-
- Van Cutsem E, Lenz HJ, Köhne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33(7):692–700. - PubMed
-
- Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–1075. - PubMed
-
- Missiaglia E, Jacobs B, D’Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014; 25(10):1995–2001. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

