Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy
- PMID: 27717298
- PMCID: PMC5648545
- DOI: 10.1056/NEJMoa1611299
Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy
Erratum in
-
Neoadjuvant PD-1 Blockade in Resectable Lung Cancer; Nivolumab and Ipilimumab in Advanced Melanoma; Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma; Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy; Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma; Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma; Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma; Rapid Eradication of a Bulky Melanoma Mass with One Dose of Immunotherapy; Genetic Basis for Clinical Response to CTLA-4 Blockade; Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma; Nivolumab plus Ipilimumab in Advanced Melanoma; Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma; Hepatotoxicity with Combination of Vemurafenib and Ipilimumab.N Engl J Med. 2018 Nov 29;379(22):2185. doi: 10.1056/NEJMx180040. Epub 2018 Nov 9. N Engl J Med. 2018. PMID: 31442371 No abstract available.
Abstract
Background: On the basis of data from a phase 2 trial that compared the checkpoint inhibitor ipilimumab at doses of 0.3 mg, 3 mg, and 10 mg per kilogram of body weight in patients with advanced melanoma, this phase 3 trial evaluated ipilimumab at a dose of 10 mg per kilogram in patients who had undergone complete resection of stage III melanoma.
Methods: After patients had undergone complete resection of stage III cutaneous melanoma, we randomly assigned them to receive ipilimumab at a dose of 10 mg per kilogram (475 patients) or placebo (476) every 3 weeks for four doses, then every 3 months for up to 3 years or until disease recurrence or an unacceptable level of toxic effects occurred. Recurrence-free survival was the primary end point. Secondary end points included overall survival, distant metastasis-free survival, and safety.
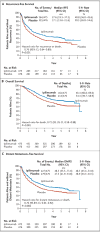
Results: At a median follow-up of 5.3 years, the 5-year rate of recurrence-free survival was 40.8% in the ipilimumab group, as compared with 30.3% in the placebo group (hazard ratio for recurrence or death, 0.76; 95% confidence interval [CI], 0.64 to 0.89; P<0.001). The rate of overall survival at 5 years was 65.4% in the ipilimumab group, as compared with 54.4% in the placebo group (hazard ratio for death, 0.72; 95.1% CI, 0.58 to 0.88; P=0.001). The rate of distant metastasis-free survival at 5 years was 48.3% in the ipilimumab group, as compared with 38.9% in the placebo group (hazard ratio for death or distant metastasis, 0.76; 95.8% CI, 0.64 to 0.92; P=0.002). Adverse events of grade 3 or 4 occurred in 54.1% of the patients in the ipilimumab group and in 26.2% of those in the placebo group. Immune-related adverse events of grade 3 or 4 occurred in 41.6% of the patients in the ipilimumab group and in 2.7% of those in the placebo group. In the ipilimumab group, 5 patients (1.1%) died owing to immune-related adverse events.
Conclusions: As adjuvant therapy for high-risk stage III melanoma, ipilimumab at a dose of 10 mg per kilogram resulted in significantly higher rates of recurrence-free survival, overall survival, and distant metastasis-free survival than placebo. There were more immune-related adverse events with ipilimumab than with placebo. (Funded by Bristol-Myers Squibb; ClinicalTrials.gov number, NCT00636168 , and EudraCT number, 2007-001974-10 .).
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures

Comment in
-
Ipilimumab Adjuvant Therapy in Melanoma.N Engl J Med. 2017 Jan 26;376(4):399. doi: 10.1056/NEJMc1615564. N Engl J Med. 2017. PMID: 28121506 No abstract available.
-
Ipilimumab Adjuvant Therapy in Melanoma.N Engl J Med. 2017 Jan 26;376(4):398. doi: 10.1056/NEJMc1615564. N Engl J Med. 2017. PMID: 28125193 No abstract available.
-
Ipilimumab Adjuvant Therapy in Melanoma.N Engl J Med. 2017 Jan 26;376(4):398-9. doi: 10.1056/NEJMc1615564. N Engl J Med. 2017. PMID: 28125194 No abstract available.
-
Estimation of Distant Metastasis-free Survival in Trials of Adjuvant Therapy for Melanoma.N Engl J Med. 2019 Apr 4;380(14):1374-1376. doi: 10.1056/NEJMc1902228. N Engl J Med. 2019. PMID: 30943345 No abstract available.
Similar articles
-
Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma.N Engl J Med. 2017 Nov 9;377(19):1824-1835. doi: 10.1056/NEJMoa1709030. Epub 2017 Sep 10. N Engl J Med. 2017. PMID: 28891423 Clinical Trial.
-
Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial.Lancet Oncol. 2015 May;16(5):522-30. doi: 10.1016/S1470-2045(15)70122-1. Epub 2015 Mar 31. Lancet Oncol. 2015. PMID: 25840693 Clinical Trial.
-
Health-related quality of life with adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): secondary outcomes of a multinational, randomised, double-blind, phase 3 trial.Lancet Oncol. 2017 Mar;18(3):393-403. doi: 10.1016/S1470-2045(17)30015-3. Epub 2017 Feb 3. Lancet Oncol. 2017. PMID: 28162999 Free PMC article. Clinical Trial.
-
Relapse-Free Survival as a Surrogate for Overall Survival in the Evaluation of Stage II-III Melanoma Adjuvant Therapy.J Natl Cancer Inst. 2018 Jan 1;110(1). doi: 10.1093/jnci/djx133. J Natl Cancer Inst. 2018. PMID: 28922786 Review.
-
Adjuvant therapy for resected stage III melanoma patients: high-dose interferon-alpha versus ipilimumab combined with kinases inhibitors.Tumori. 2012 Mar-Apr;98(2):185-90. doi: 10.1177/030089161209800202. Tumori. 2012. PMID: 22677983 Review.
Cited by
-
Annals of Surgical Oncology Practice Guidelines Series: Adjuvant and Neoadjuvant Therapy for Melanoma.Ann Surg Oncol. 2025 Jan;32(1):3-11. doi: 10.1245/s10434-024-16418-y. Epub 2024 Nov 4. Ann Surg Oncol. 2025. PMID: 39495363 Review.
-
Key Determinants of Immune-Mediated Adverse Reactions to Oncology Drugs.Cancers (Basel). 2023 Nov 28;15(23):5622. doi: 10.3390/cancers15235622. Cancers (Basel). 2023. PMID: 38067327 Free PMC article. Review.
-
Management of Immune Checkpoint Inhibitor Toxicities.Cancer Manag Res. 2020 Sep 28;12:9139-9158. doi: 10.2147/CMAR.S218756. eCollection 2020. Cancer Manag Res. 2020. PMID: 33061607 Free PMC article. Review.
-
Targeting TIGIT for cancer immunotherapy: recent advances and future directions.Biomark Res. 2024 Jan 16;12(1):7. doi: 10.1186/s40364-023-00543-z. Biomark Res. 2024. PMID: 38229100 Free PMC article. Review.
-
Efficacy of immune checkpoint inhibitors in different types of melanoma.Hum Vaccin Immunother. 2021 Jan 2;17(1):4-13. doi: 10.1080/21645515.2020.1771986. Epub 2020 Jul 14. Hum Vaccin Immunother. 2021. PMID: 32663057 Free PMC article.
References
-
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. - PubMed
-
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–64. - PubMed
-
- Eggermont AMM, Spatz A, Robert C. Cutaneous melanoma. Lancet. 2014;383:816–27. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
