Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination
- PMID: 27702895
- PMCID: PMC5086995
- DOI: 10.1073/pnas.1606050113
Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination
Abstract
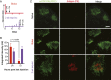
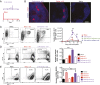
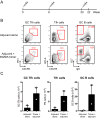
Natural infections expose the immune system to escalating antigen and inflammation over days to weeks, whereas nonlive vaccines are single bolus events. We explored whether the immune system responds optimally to antigen kinetics most similar to replicating infections, rather than a bolus dose. Using HIV antigens, we found that administering a given total dose of antigen and adjuvant over 1-2 wk through repeated injections or osmotic pumps enhanced humoral responses, with exponentially increasing (exp-inc) dosing profiles eliciting >10-fold increases in antibody production relative to bolus vaccination post prime. Computational modeling of the germinal center response suggested that antigen availability as higher-affinity antibodies evolve enhances antigen capture in lymph nodes. Consistent with these predictions, we found that exp-inc dosing led to prolonged antigen retention in lymph nodes and increased Tfh cell and germinal center B-cell numbers. Thus, regulating the antigen and adjuvant kinetics may enable increased vaccine potency.
Keywords: antigen retention; computational immunology; germinal center formation; humoral response; vaccination kinetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The role of follicular helper T cells and the germinal center in HIV-1 gp120 DNA prime and gp120 protein boost vaccination.Hum Vaccin Immunother. 2014;10(7):1985-92. doi: 10.4161/hv.28659. Hum Vaccin Immunother. 2014. PMID: 25424808 Free PMC article.
-
Divergent Primary Immune Responses Induced by Human Immunodeficiency Virus-1 gp120 and Hepatitis B Surface Antigen Determine Antibody Recall Responses.Virol Sin. 2018 Dec;33(6):502-514. doi: 10.1007/s12250-018-0074-6. Epub 2018 Dec 19. Virol Sin. 2018. PMID: 30569292 Free PMC article.
-
Germinal center enhancement by extended antigen availability.Curr Opin Immunol. 2017 Aug;47:64-69. doi: 10.1016/j.coi.2017.06.008. Epub 2017 Jul 21. Curr Opin Immunol. 2017. PMID: 28738289 Free PMC article. Review.
-
Beraprost enhances production of antigen-specific IgG isotypes without modulating germinal center B cell generation and the affinity maturation.Int Immunopharmacol. 2013 Apr;15(4):735-42. doi: 10.1016/j.intimp.2013.03.004. Epub 2013 Mar 15. Int Immunopharmacol. 2013. PMID: 23499642
-
[Adjuvants as factors improving efficiency of vaccination].Postepy Hig Med Dosw (Online). 2004 Mar 2;58:47-59. Postepy Hig Med Dosw (Online). 2004. PMID: 15069382 Review. Polish.
Cited by
-
Advanced technologies for the development of infectious disease vaccines.Nat Rev Drug Discov. 2024 Dec;23(12):914-938. doi: 10.1038/s41573-024-01041-z. Epub 2024 Oct 21. Nat Rev Drug Discov. 2024. PMID: 39433939 Review.
-
Co-Anchoring of Engineered Immunogen and Immunostimulatory Cytokines to Alum Promotes Enhanced-Humoral Immunity.Adv Ther (Weinh). 2022 Jul;5(7):2100235. doi: 10.1002/adtp.202100235. Epub 2022 Apr 7. Adv Ther (Weinh). 2022. PMID: 36311814 Free PMC article.
-
Technological approaches to streamline vaccination schedules, progressing towards single-dose vaccines.NPJ Vaccines. 2020 Sep 18;5:88. doi: 10.1038/s41541-020-00238-8. eCollection 2020. NPJ Vaccines. 2020. PMID: 33024579 Free PMC article. Review.
-
Engineering early memory B-cell-like phenotype in hydrogel-based immune organoids.J Biomed Mater Res A. 2022 Aug;110(8):1435-1447. doi: 10.1002/jbm.a.37388. Epub 2022 Apr 7. J Biomed Mater Res A. 2022. PMID: 35388946 Free PMC article.
-
Protein Nanoparticle-Mediated Delivery of Recombinant Influenza Hemagglutinin Enhances Immunogenicity and Breadth of the Antibody Response.ACS Infect Dis. 2023 Feb 10;9(2):239-252. doi: 10.1021/acsinfecdis.2c00362. Epub 2023 Jan 6. ACS Infect Dis. 2023. PMID: 36607269 Free PMC article.
References
-
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19(12):1597–1608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

