Isotope-targeted glycoproteomics (IsoTaG) analysis of sialylated N- and O-glycopeptides on an Orbitrap Fusion Tribrid using azido and alkynyl sugars
- PMID: 27695962
- PMCID: PMC5342897
- DOI: 10.1007/s00216-016-9934-9
Isotope-targeted glycoproteomics (IsoTaG) analysis of sialylated N- and O-glycopeptides on an Orbitrap Fusion Tribrid using azido and alkynyl sugars
Abstract
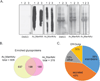
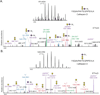
Protein glycosylation is a post-translational modification (PTM) responsible for many aspects of proteomic diversity and biological regulation. Assignment of intact glycan structures to specific protein attachment sites is a critical step towards elucidating the function encoded in the glycome. Previously, we developed isotope-targeted glycoproteomics (IsoTaG) as a mass-independent mass spectrometry method to characterize azide-labeled intact glycopeptides from complex proteomes. Here, we extend the IsoTaG approach with the use of alkynyl sugars as metabolic labels and employ new probes in analysis of the sialylated glycoproteome from PC-3 cells. Using an Orbitrap Fusion Tribrid mass spectrometer, we identified 699 intact glycopeptides from 192 glycoproteins. These intact glycopeptides represent a total of eight sialylated glycan structures across 126 N- and 576 O-glycopeptides. IsoTaG is therefore an effective platform for identification of intact glycopeptides labeled by alkynyl or azido sugars and will facilitate further studies of the glycoproteome.
Keywords: Chemical proteomics; Glycoproteomics; LC-MS/MS; Metabolic labeling; Sialic acid.
Conflict of interest statement
Figures

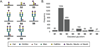
Similar articles
-
Isotope Targeted Glycoproteomics (IsoTaG) to Characterize Intact, Metabolically Labeled Glycopeptides from Complex Proteomes.Curr Protoc Chem Biol. 2016 Mar 16;8(1):59-82. doi: 10.1002/9780470559277.ch150185. Curr Protoc Chem Biol. 2016. PMID: 26995354 Free PMC article.
-
Isotope-targeted glycoproteomics (IsoTaG): a mass-independent platform for intact N- and O-glycopeptide discovery and analysis.Nat Methods. 2015 Jun;12(6):561-7. doi: 10.1038/nmeth.3366. Epub 2015 Apr 20. Nat Methods. 2015. PMID: 25894945 Free PMC article.
-
Development of IsoTaG, a Chemical Glycoproteomics Technique for Profiling Intact N- and O-Glycopeptides from Whole Cell Proteomes.J Proteome Res. 2017 Apr 7;16(4):1706-1718. doi: 10.1021/acs.jproteome.6b01053. Epub 2017 Feb 28. J Proteome Res. 2017. PMID: 28244757 Free PMC article.
-
Towards structure-focused glycoproteomics.Biochem Soc Trans. 2021 Feb 26;49(1):161-186. doi: 10.1042/BST20200222. Biochem Soc Trans. 2021. PMID: 33439247 Free PMC article. Review.
-
Current Technologies for Complex Glycoproteomics and Their Applications to Biology/Disease-Driven Glycoproteomics.J Proteome Res. 2018 Dec 7;17(12):4097-4112. doi: 10.1021/acs.jproteome.8b00515. Epub 2018 Oct 25. J Proteome Res. 2018. PMID: 30359034 Review.
Cited by
-
Minimalist Tetrazine N-Acetyl Muramic Acid Probes for Rapid and Efficient Labeling of Commensal and Pathogenic Peptidoglycans in Living Bacterial Culture and During Macrophage Invasion.J Am Chem Soc. 2024 Mar 13;146(10):6817-6829. doi: 10.1021/jacs.3c13644. Epub 2024 Mar 1. J Am Chem Soc. 2024. PMID: 38427023
-
Mass Spectrometry-Based Techniques to Elucidate the Sugar Code.Chem Rev. 2022 Apr 27;122(8):7840-7908. doi: 10.1021/acs.chemrev.1c00380. Epub 2021 Sep 7. Chem Rev. 2022. PMID: 34491038 Free PMC article. Review.
-
Dismantling the bacterial glycocalyx: Chemical tools to probe, perturb, and image bacterial glycans.Bioorg Med Chem. 2021 Jul 15;42:116268. doi: 10.1016/j.bmc.2021.116268. Epub 2021 Jun 7. Bioorg Med Chem. 2021. PMID: 34130219 Free PMC article. Review.
-
A Pragmatic Guide to Enrichment Strategies for Mass Spectrometry-Based Glycoproteomics.Mol Cell Proteomics. 2021;20:100029. doi: 10.1074/mcp.R120.002277. Epub 2020 Dec 20. Mol Cell Proteomics. 2021. PMID: 33583771 Free PMC article. Review.
-
Recent Advances in Analytical Approaches for Glycan and Glycopeptide Quantitation.Mol Cell Proteomics. 2021;20:100054. doi: 10.1074/mcp.R120.002095. Epub 2021 Feb 20. Mol Cell Proteomics. 2021. PMID: 32576592 Free PMC article. Review.
References
-
- Amon R, Reuven EM, Leviatan Ben-Arye S, Padler-Karavani V. Glycans in immune recognition and response. Carbohydr Res. 2014;389:115. - PubMed
-
- Adamczyk B, Tharmalingam T, Rudd PM. Glycans as cancer biomarkers. Biochim Biophys Acta. 2012;1820(9):1347. - PubMed
-
- Paszek MJ, DuFort CC, Rossier O, Bainer R, Mouw JK, Godula K, Hudak JE, Lakins JN, Wijekoon AC, Cassereau L, Rubashkin MG, Magbanua MJ, Thorn KS, Davidson MW, Rugo HS, Park JW, Hammer DA, Giannone G, Bertozzi CR, Weaver VM. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. 2014;511:319. - PMC - PubMed
-
- Brown JR, Fuster MM, Li R, Varki N, Glass CA, Esko JD. A disaccharide-based inhibitor of glycosylation attenuates metastatic tumor cell dissemination. Clin Cancer Res. 2006;12(9):2894. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

