A Role for Human N-alpha Acetyltransferase 30 (Naa30) in Maintaining Mitochondrial Integrity
- PMID: 27694331
- PMCID: PMC5098035
- DOI: 10.1074/mcp.M116.061010
A Role for Human N-alpha Acetyltransferase 30 (Naa30) in Maintaining Mitochondrial Integrity
Abstract
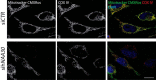
N-terminal acetylation (Nt-acetylation) by N-terminal acetyltransferases (NATs) is one of the most common protein modifications in eukaryotes. The NatC complex represents one of three major NATs of which the substrate profile remains largely unexplored. Here, we defined the in vivo human NatC Nt-acetylome on a proteome-wide scale by combining knockdown of its catalytic subunit Naa30 with positional proteomics. We identified 46 human NatC substrates, expanding our current knowledge on the substrate repertoire of NatC which now includes proteins harboring Met-Leu, Met-Ile, Met-Phe, Met-Trp, Met-Val, Met-Met, Met-His and Met-Lys N termini. Upon Naa30 depletion the expression levels of several organellar proteins were found reduced, in particular mitochondrial proteins, some of which were found to be NatC substrates. Interestingly, knockdown of Naa30 induced the loss of mitochondrial membrane potential and fragmentation of mitochondria. In conclusion, NatC Nt-acetylates a large variety of proteins and is essential for mitochondrial integrity and function.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Co-translational, Post-translational, and Non-catalytic Roles of N-Terminal Acetyltransferases.Mol Cell. 2019 Mar 21;73(6):1097-1114. doi: 10.1016/j.molcel.2019.02.007. Epub 2019 Mar 13. Mol Cell. 2019. PMID: 30878283 Free PMC article. Review.
-
Expanded in vivo substrate profile of the yeast N-terminal acetyltransferase NatC.J Biol Chem. 2023 Feb;299(2):102824. doi: 10.1016/j.jbc.2022.102824. Epub 2022 Dec 22. J Biol Chem. 2023. PMID: 36567016 Free PMC article.
-
Identification of an alternatively spliced nuclear isoform of human N-terminal acetyltransferase Naa30.Gene. 2018 Feb 20;644:27-37. doi: 10.1016/j.gene.2017.12.019. Epub 2017 Dec 13. Gene. 2018. PMID: 29247799
-
A nonsense variant in the N-terminal acetyltransferase NAA30 may be associated with global developmental delay and tracheal cleft.Am J Med Genet A. 2023 Sep;191(9):2402-2410. doi: 10.1002/ajmg.a.63338. Epub 2023 Jun 30. Am J Med Genet A. 2023. PMID: 37387332
-
N-acetyltransferase and inflammation: Bridging an unexplored niche.Gene. 2023 Dec 15;887:147730. doi: 10.1016/j.gene.2023.147730. Epub 2023 Aug 23. Gene. 2023. PMID: 37625560 Review.
Cited by
-
Molecular mechanism of N-terminal acetylation by the ternary NatC complex.Structure. 2021 Oct 7;29(10):1094-1104.e4. doi: 10.1016/j.str.2021.05.003. Epub 2021 May 20. Structure. 2021. PMID: 34019809 Free PMC article.
-
Biallelic NAA60 variants with impaired n-terminal acetylation capacity cause autosomal recessive primary familial brain calcifications.Nat Commun. 2024 Mar 13;15(1):2269. doi: 10.1038/s41467-024-46354-0. Nat Commun. 2024. PMID: 38480682 Free PMC article.
-
Divergent architecture of the heterotrimeric NatC complex explains N-terminal acetylation of cognate substrates.Nat Commun. 2020 Nov 2;11(1):5506. doi: 10.1038/s41467-020-19321-8. Nat Commun. 2020. PMID: 33139728 Free PMC article.
-
N-terminal acetylation shields proteins from degradation and promotes age-dependent motility and longevity.Nat Commun. 2023 Oct 27;14(1):6774. doi: 10.1038/s41467-023-42342-y. Nat Commun. 2023. PMID: 37891180 Free PMC article.
-
Co-translational, Post-translational, and Non-catalytic Roles of N-Terminal Acetyltransferases.Mol Cell. 2019 Mar 21;73(6):1097-1114. doi: 10.1016/j.molcel.2019.02.007. Epub 2019 Mar 13. Mol Cell. 2019. PMID: 30878283 Free PMC article. Review.
References
-
- Arnesen T., Van Damme P., Polevoda B., Helsens K., Evjenth R., Colaert N., Varhaug J. E., Vandekerckhove J., Lillehaug J. R., Sherman F., and Gevaert K. (2009) Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. Proc. Natl. Acad. Sci. USA 106, 8157–8162 - PMC - PubMed
-
- Brown J. L., and Roberts W. K. (1976) Evidence that approximately eighty percent of the soluble proteins from ehrlich ascites cells are IV-acetylated. J. Biol. Chem. 251, 1009–1014 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

