Defining a novel leptin-melanocortin-kisspeptin pathway involved in the metabolic control of puberty
- PMID: 27688998
- PMCID: PMC5034608
- DOI: 10.1016/j.molmet.2016.08.003
Defining a novel leptin-melanocortin-kisspeptin pathway involved in the metabolic control of puberty
Abstract
Objective: Puberty is a key developmental phenomenon highly sensitive to metabolic modulation. Worrying trends of changes in the timing of puberty have been reported in humans. These might be linked to the escalating prevalence of childhood obesity and could have deleterious impacts on later (cardio-metabolic) health, but their underlying mechanisms remain unsolved. The neuropeptide α-MSH, made by POMC neurons, plays a key role in energy homeostasis by mediating the actions of leptin and likely participates in the control of reproduction. However, its role in the metabolic regulation of puberty and interplay with kisspeptin, an essential puberty-regulating neuropeptide encoded by Kiss1, remain largely unknown. We aim here to unveil the potential contribution of central α-MSH signaling in the metabolic control of puberty by addressing its role in mediating the pubertal effects of leptin and its potential interaction with kisspeptin.
Methods: Using wild type and genetically modified rodent models, we implemented pharmacological studies, expression analyses, electrophysiological recordings, and virogenetic approaches involving DREADD technology to selectively inhibit Kiss1 neurons, in order to interrogate the physiological role of a putative leptin→α-MSH→kisspeptin pathway in the metabolic control of puberty.
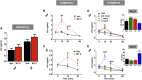
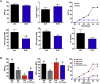
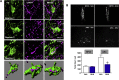
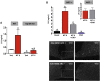
Results: Stimulation of central α-MSH signaling robustly activated the reproductive axis in pubertal rats, whereas chronic inhibition of melanocortin receptors MC3/4R, delayed puberty, and prevented the permissive effect of leptin on puberty onset. Central blockade of MC3/4R or genetic elimination of kisspeptin receptors from POMC neurons did not affect kisspeptin effects. Conversely, congenital ablation of kisspeptin receptors or inducible, DREADD-mediated inhibition of arcuate nucleus (ARC) Kiss1 neurons resulted in markedly attenuated gonadotropic responses to MC3/4R activation. Furthermore, close appositions were observed between POMC fibers and ARC Kiss1 neurons while blockade of α-MSH signaling suppressed Kiss1 expression in the ARC of pubertal rats.
Conclusions: Our physiological, virogenetic, and functional genomic studies document a novel α-MSH→kisspeptin→GnRH neuronal signaling pathway involved in transmitting the permissive effects of leptin on pubertal maturation, which is relevant for the metabolic (and, eventually, pharmacological) regulation of puberty onset.
Keywords: DREADDs; Kisspeptin; Leptin; Metabolism; Puberty; α-MSH.
Figures

Similar articles
-
Connecting nutritional deprivation and pubertal inhibition via GRK2-mediated repression of kisspeptin actions in GnRH neurons.Metabolism. 2022 Apr;129:155141. doi: 10.1016/j.metabol.2022.155141. Epub 2022 Jan 22. Metabolism. 2022. PMID: 35074314 Free PMC article.
-
Metabolic regulation of female puberty via hypothalamic AMPK-kisspeptin signaling.Proc Natl Acad Sci U S A. 2018 Nov 6;115(45):E10758-E10767. doi: 10.1073/pnas.1802053115. Epub 2018 Oct 22. Proc Natl Acad Sci U S A. 2018. PMID: 30348767 Free PMC article.
-
Early metabolic programming of puberty onset: impact of changes in postnatal feeding and rearing conditions on the timing of puberty and development of the hypothalamic kisspeptin system.Endocrinology. 2011 Sep;152(9):3396-408. doi: 10.1210/en.2010-1415. Epub 2011 Jun 28. Endocrinology. 2011. PMID: 21712362
-
Metabolic control of puberty: roles of leptin and kisspeptins.Horm Behav. 2013 Jul;64(2):187-94. doi: 10.1016/j.yhbeh.2013.01.014. Horm Behav. 2013. PMID: 23998663 Review.
-
Kisspeptin and energy balance in reproduction.Reproduction. 2014 Feb 3;147(3):R53-63. doi: 10.1530/REP-13-0509. Print 2014 Mar. Reproduction. 2014. PMID: 24327738 Review.
Cited by
-
Beyond the Metabolic Syndrome: Non-Obvious Complications of Obesity in Children.Children (Basel). 2023 Dec 9;10(12):1905. doi: 10.3390/children10121905. Children (Basel). 2023. PMID: 38136107 Free PMC article. Review.
-
Central growth hormone signaling is not required for the timing of puberty.J Endocrinol. 2019 Aug 1:JOE-19-0242.R1. doi: 10.1530/JOE-19-0242. Online ahead of print. J Endocrinol. 2019. PMID: 31470413 Free PMC article.
-
Nasal delivery of nerve growth factor rescue hypogonadism by up-regulating GnRH and testosterone in aging male mice.EBioMedicine. 2018 Sep;35:295-306. doi: 10.1016/j.ebiom.2018.08.021. Epub 2018 Aug 18. EBioMedicine. 2018. PMID: 30131307 Free PMC article.
-
Genes underlying delayed puberty.Mol Cell Endocrinol. 2018 Nov 15;476:119-128. doi: 10.1016/j.mce.2018.05.001. Epub 2018 May 4. Mol Cell Endocrinol. 2018. PMID: 29730183 Free PMC article. Review.
-
Metabolic Impact on the Hypothalamic Kisspeptin-Kiss1r Signaling Pathway.Front Endocrinol (Lausanne). 2018 Mar 28;9:123. doi: 10.3389/fendo.2018.00123. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29643834 Free PMC article. Review.
References
-
- Parent A.S., Teilmann G., Juul A., Skakkebaek N.E., Toppari J., Bourguignon J.P. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocrine Reviews. 2003;24:668–693. - PubMed
-
- De Leonibus C., Marcovecchio M.L., Chiavaroli V., de Giorgis T., Chiarelli F., Mohn A. Timing of puberty and physical growth in obese children: a longitudinal study in boys and girls. Pediatric Obesity. 2013;9:292–299. - PubMed
-
- Lakshman R., Forouhi N.G., Sharp S.J., Luben R., Bingham S.A., Khaw K.T. Early age at menarche associated with cardiovascular disease and mortality. The Journal of Clinical Endocrinology and Metabolism. 2009;94:4953–4960. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

