Palmitoyl acyltransferase DHHC21 mediates endothelial dysfunction in systemic inflammatory response syndrome
- PMID: 27653213
- PMCID: PMC5036164
- DOI: 10.1038/ncomms12823
Palmitoyl acyltransferase DHHC21 mediates endothelial dysfunction in systemic inflammatory response syndrome
Abstract
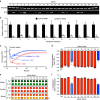
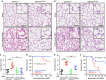
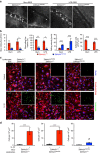
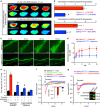
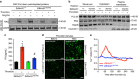
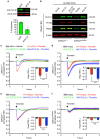
Endothelial dysfunction is a hallmark of systemic inflammatory response underlying multiple organ failure. Here we report a novel function of DHHC-containing palmitoyl acyltransferases (PATs) in mediating endothelial inflammation. Pharmacological inhibition of PATs attenuates barrier leakage and leucocyte adhesion induced by endothelial junction hyperpermeability and ICAM-1 expression during inflammation. Among 11 DHHCs detected in vascular endothelium, DHHC21 is required for barrier response. Mice with DHHC21 function deficiency (Zdhhc21dep/dep) exhibit marked resistance to injury, characterized by reduced plasma leakage, decreased leucocyte adhesion and ameliorated lung pathology, culminating in improved survival. Endothelial cells from Zdhhc21dep/dep display blunted barrier dysfunction and leucocyte adhesion, whereas leucocytes from these mice did not show altered adhesiveness. Furthermore, inflammation enhances PLCβ1 palmitoylation and signalling activity, effects significantly reduced in Zdhhc21dep/dep and rescued by DHHC21 overexpression. Likewise, overexpression of wild-type, not mutant, PLCβ1 augments barrier dysfunction. Altogether, these data suggest the involvement of DHHC21-mediated PLCβ1 palmitoylation in endothelial inflammation.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
Similar articles
-
DHHC21 deficiency attenuates renal dysfunction during septic injury.Sci Rep. 2021 May 27;11(1):11146. doi: 10.1038/s41598-021-89983-x. Sci Rep. 2021. PMID: 34045489 Free PMC article.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Induction of PD-1 and CD44 in CD4+ T cells by circulatory extracellular vesicles from severe dengue patients drives endothelial damage via the NF-kB signaling pathway.J Virol. 2024 Dec 31:e0186124. doi: 10.1128/jvi.01861-24. Online ahead of print. J Virol. 2024. PMID: 39745465
-
Endothelial Dysfunction in Obesity and Therapeutic Targets.Adv Exp Med Biol. 2024;1460:489-538. doi: 10.1007/978-3-031-63657-8_17. Adv Exp Med Biol. 2024. PMID: 39287863 Review.
-
Conservative, physical and surgical interventions for managing faecal incontinence and constipation in adults with central neurological diseases.Cochrane Database Syst Rev. 2024 Oct 29;10(10):CD002115. doi: 10.1002/14651858.CD002115.pub6. Cochrane Database Syst Rev. 2024. PMID: 39470206
Cited by
-
Blocking Palmitoylation of Apelin Receptor Alleviates Morphine Tolerance in Neuropathic Cancer Pain.Int J Biol Sci. 2024 Jan 1;20(1):47-60. doi: 10.7150/ijbs.86888. eCollection 2024. Int J Biol Sci. 2024. PMID: 38164190 Free PMC article.
-
Protein palmitoylation regulates extracellular vesicle production and function in sepsis.J Extracell Biol. 2022 Jul;1(7):e50. doi: 10.1002/jex2.50. Epub 2022 Jul 5. J Extracell Biol. 2022. PMID: 38419739 Free PMC article.
-
Emerging strategies for treating autoimmune disease with genetically modified dendritic cells.Cell Commun Signal. 2024 May 7;22(1):262. doi: 10.1186/s12964-024-01641-7. Cell Commun Signal. 2024. PMID: 38715122 Free PMC article. Review.
-
S-acylation of P2K1 mediates extracellular ATP-induced immune signaling in Arabidopsis.Nat Commun. 2021 May 12;12(1):2750. doi: 10.1038/s41467-021-22854-1. Nat Commun. 2021. PMID: 33980819 Free PMC article.
-
PRE-084 as a tool to uncover potential therapeutic applications for selective sigma-1 receptor activation.Int J Biochem Cell Biol. 2020 Sep;126:105803. doi: 10.1016/j.biocel.2020.105803. Epub 2020 Jul 12. Int J Biochem Cell Biol. 2020. PMID: 32668330 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

