A global review of national influenza immunization policies: Analysis of the 2014 WHO/UNICEF Joint Reporting Form on immunization
- PMID: 27646030
- PMCID: PMC5357765
- DOI: 10.1016/j.vaccine.2016.07.045
A global review of national influenza immunization policies: Analysis of the 2014 WHO/UNICEF Joint Reporting Form on immunization
Abstract
Introduction: The WHO recommends annual influenza vaccination to prevent influenza illness in high-risk groups. Little is known about national influenza immunization policies globally.
Material and methods: The 2014 WHO/UNICEF Joint Reporting Form (JRF) on Immunization was adapted to capture data on influenza immunization policies. We combined this dataset with additional JRF information on new vaccine introductions and strength of immunization programmes, as well as publicly available data on country economic status. Data from countries that did not complete the JRF were sought through additional sources. We described data on country influenza immunization policies and used bivariate analyses to identify factors associated with having such policies.
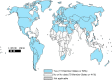
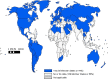
Results: Of 194 WHO Member States, 115 (59%) reported having a national influenza immunization policy in 2014. Among countries with a national policy, programmes target specific WHO-defined risk groups, including pregnant women (42%), young children (28%), adults with chronic illnesses (46%), the elderly (45%), and health care workers (47%). The Americas, Europe, and Western Pacific were the WHO regions that had the highest percentages of countries reporting that they had national influenza immunization policies. Compared to countries without policies, countries with policies were significantly more likely to have the following characteristics: to be high or upper middle income (p<0.0001); to have introduced birth dose hepatitis B virus vaccine (p<0.0001), pneumococcal conjugate vaccine (p=0.032), or human papilloma virus vaccine (p=0.002); to have achieved global goals for diphtheria-tetanus-pertussis vaccine coverage (p<0.0001); and to have a functioning National Immunization Technical Advisory Group (p<0.0001).
Conclusions: The 2014 revision of the JRF permitted a global assessment of national influenza immunization policies. The 59% of countries reporting that they had policies are wealthier, use more new or under-utilized vaccines, and have stronger immunization systems. Addressing disparities in public health resources and strengthening immunization systems may facilitate influenza vaccine introduction and use.
Keywords: Immunization programmes; Influenza vaccines; Joint Reporting Form; Vaccination; World Health Organization.
Copyright © 2016. Published by Elsevier Ltd.
Figures
Similar articles
-
Influenza vaccination in the Americas: Progress and challenges after the 2009 A(H1N1) influenza pandemic.Hum Vaccin Immunother. 2016 Aug 2;12(8):2206-2214. doi: 10.1080/21645515.2016.1157240. Epub 2016 May 19. Hum Vaccin Immunother. 2016. PMID: 27196006 Free PMC article.
-
A global review of seasonal influenza vaccine introduction: analysis of the WHO/UNICEF Joint Reporting Form.Expert Rev Vaccines. 2019 Aug;18(8):859-865. doi: 10.1080/14760584.2019.1640119. Epub 2019 Jul 18. Expert Rev Vaccines. 2019. PMID: 31318303
-
National vaccination policies for health workers - A cross-sectional global overview.Vaccine. 2024 Feb 6;42(4):757-769. doi: 10.1016/j.vaccine.2023.04.083. Epub 2023 Jun 14. Vaccine. 2024. PMID: 37321897
-
A review of policies and coverage of seasonal influenza vaccination programs in the WHO Eastern Mediterranean Region.Influenza Other Respir Viruses. 2023 Mar 21;17(3):e13126. doi: 10.1111/irv.13126. eCollection 2023 Mar. Influenza Other Respir Viruses. 2023. PMID: 36970569 Free PMC article. Review.
-
Seasonal influenza vaccine dose distribution in 157 countries (2004-2011).Vaccine. 2014 Nov 12;32(48):6369-76. doi: 10.1016/j.vaccine.2014.07.012. Epub 2014 Nov 1. Vaccine. 2014. PMID: 25442403 Review.
Cited by
-
Informing the public health response to COVID-19: a systematic review of risk factors for disease, severity, and mortality.BMC Infect Dis. 2021 Apr 12;21(1):342. doi: 10.1186/s12879-021-05992-1. BMC Infect Dis. 2021. PMID: 33845766 Free PMC article.
-
Impact of Maternal HIV Infection and Placental Malaria on the Transplacental Transfer of Influenza Antibodies in Mother-Infant Pairs in Malawi, 2013-2014.Open Forum Infect Dis. 2019 Aug 28;6(10):ofz383. doi: 10.1093/ofid/ofz383. eCollection 2019 Oct. Open Forum Infect Dis. 2019. PMID: 31660347 Free PMC article.
-
Cost-effectiveness of seasonal influenza vaccination of children in China: a modeling analysis.Infect Dis Poverty. 2023 Oct 11;12(1):92. doi: 10.1186/s40249-023-01144-6. Infect Dis Poverty. 2023. PMID: 37821942 Free PMC article.
-
Pandemic Flu, 1918: After hundred years, India is as vulnerable.Indian J Med Res. 2018 Mar;147(3):221-224. doi: 10.4103/ijmr.IJMR_407_18. Indian J Med Res. 2018. PMID: 29923508 Free PMC article. No abstract available.
-
Public health and economic benefits of seasonal influenza vaccination in risk groups in France, Italy, Spain and the UK: state of play and perspectives.BMC Public Health. 2024 May 3;24(1):1222. doi: 10.1186/s12889-024-18694-5. BMC Public Health. 2024. PMID: 38702667 Free PMC article.
References
-
- World Health Organization . World Health Organization; Geneva: 2016. Global action plan for influenza vaccines.
-
- Collin N., de Radigues X. Vaccine production capacity for seasonal and pandemic (H1N1) 2009 influenza. Vaccine. 2009;27:5184–5186. - PubMed
-
- Partridge J., Kieny M.P. Global production of seasonal and pandemic (H1N1) influenza vaccines in 2009–2010 and comparison with previous estimates and global action plan targets. Vaccine. 2010;28:4709–4712. - PubMed
-
- Partridge J., Kieny M.P. Global production capacity of seasonal influenza vaccine in 2011. Vaccine. 2013;31:728–731. - PubMed
-
- McLean KA, Goldin S, Nannei C, Sparrow E, Torelli G. The 2015 global production capacity of seasonal and pandemic influenza vaccine. Vaccine. 2016;34:5410–5413. http://dx.doi.org/2016.10.1016/j.vaccine.2016.08.019. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

