IDH1 and IDH2 mutations as novel therapeutic targets: current perspectives
- PMID: 27621679
- PMCID: PMC5015873
- DOI: 10.2147/JBM.S70716
IDH1 and IDH2 mutations as novel therapeutic targets: current perspectives
Abstract
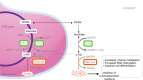
Isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2) are key metabolic enzymes that convert isocitrate to α-ketoglutarate. IDH1/2 mutations define distinct subsets of cancers, including low-grade gliomas and secondary glioblastomas, chondrosarcomas, intrahepatic cholangiocarcinomas, and hematologic malignancies. Somatic point mutations in IDH1/2 confer a gain-of-function in cancer cells, resulting in the accumulation and secretion in vast excess of an oncometabolite, the D-2-hydroxyglutarate (D-2HG). Overproduction of D-2HG interferes with cellular metabolism and epigenetic regulation, contributing to oncogenesis. Indeed, high levels of D-2HG inhibit α-ketoglutarate-dependent dioxygenases, including histone and DNA demethylases, leading to histone and DNA hypermethylation and finally a block in cell differentiation. Furthermore, D-2HG is a biomarker suitable for the detection of IDH1/2 mutations at diagnosis and predictive of the clinical response. Finally, mutant-IDH1/2 enzymes inhibitors have entered clinical trials for patients with IDH1/2 mutations and represent a novel drug class for targeted therapy.
Keywords: 2-HG; IDH1; IDH2; acute myeloid leukemia; epigenetic; glioma; oncogene; targeted therapies; tumor metabolism.
Figures
Similar articles
-
Ivosidenib to treat adult patients with relapsed or refractory acute myeloid leukemia.Drugs Today (Barc). 2020 Jan;56(1):21-32. doi: 10.1358/dot.2020.56.1.3078363. Drugs Today (Barc). 2020. PMID: 32055803
-
Isocitrate dehydrogenase gene mutations and 2-hydroxyglutarate accumulation in esophageal squamous cell carcinoma.Med Oncol. 2018 Nov 30;36(1):11. doi: 10.1007/s12032-018-1229-x. Med Oncol. 2018. PMID: 30506321
-
IDH mutations in cancer and progress toward development of targeted therapeutics.Ann Oncol. 2016 Apr;27(4):599-608. doi: 10.1093/annonc/mdw013. Ann Oncol. 2016. PMID: 27005468 Review.
-
Isocitrate dehydrogenase mutation as a therapeutic target in gliomas.Chin Clin Oncol. 2017 Jun;6(3):33. doi: 10.21037/cco.2017.06.11. Chin Clin Oncol. 2017. PMID: 28705010
-
Targeting isocitrate dehydrogenase (IDH) in cancer.Discov Med. 2016 May;21(117):373-80. Discov Med. 2016. PMID: 27355333 Review.
Cited by
-
Generation of induced neural stem cells with inducible IDH1R132H for analysis of glioma development and drug testing.PLoS One. 2020 Sep 18;15(9):e0239325. doi: 10.1371/journal.pone.0239325. eCollection 2020. PLoS One. 2020. PMID: 32946483 Free PMC article.
-
A Case of Metastatic Biliary Tract Cancer Diagnosed Through Identification of an IDH1 Mutation.Oncologist. 2019 Feb;24(2):151-156. doi: 10.1634/theoncologist.2018-0210. Epub 2018 Oct 23. Oncologist. 2019. PMID: 30352944 Free PMC article.
-
Engrailed 1 overexpression as a potential prognostic marker in Lower Grade Glioma.PeerJ. 2019 Sep 16;7:e7414. doi: 10.7717/peerj.7414. eCollection 2019. PeerJ. 2019. PMID: 31576231 Free PMC article.
-
Rescue of TCA Cycle Dysfunction for Cancer Therapy.J Clin Med. 2019 Dec 6;8(12):2161. doi: 10.3390/jcm8122161. J Clin Med. 2019. PMID: 31817761 Free PMC article. Review.
-
Ivosidenib: First Global Approval.Drugs. 2018 Sep;78(14):1509-1516. doi: 10.1007/s40265-018-0978-3. Drugs. 2018. PMID: 30209701 Free PMC article. Review.
References
-
- Sjöblom T, Jones S, Wood LD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314(5797):268–274. - PubMed
-
- Kosmider O, Gelsi-Boyer V, Slama L, et al. Mutations of IDH1 and IDH2 genes in early and accelerated phases of myelodysplastic syndromes and MDS/myeloproliferative neoplasms. Leukemia. 2010;24(5):1094–1096. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

