N-terminal fragment of cardiac myosin binding protein-C triggers pro-inflammatory responses in vitro
- PMID: 27616755
- PMCID: PMC5107329
- DOI: 10.1016/j.yjmcc.2016.09.003
N-terminal fragment of cardiac myosin binding protein-C triggers pro-inflammatory responses in vitro
Abstract
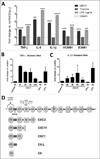
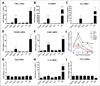
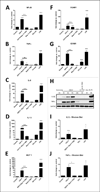
Myocardial infarction (MI) leads to loss and degradation of contractile cardiac tissue followed by sterile inflammation of the myocardium through activation and recruitment of innate and adaptive cells of the immune system. Recently, it was shown that cardiac myosin binding protein-C (cMyBP-C), a protein of the cardiac sarcomere, is degraded following MI, releasing a predominant N-terminal 40-kDa fragment (C0C1f) into myocardial tissue and the systemic circulation. We hypothesized that early release of C0C1f contributes to the initiation of inflammation and plays a key role in recruitment and activation of immune cells. Therefore, we investigated the role of C0C1f on macrophage/monocyte activation using both mouse bone marrow-derived macrophages and human monocytes. Here we demonstrate that C0C1f leads to macrophage/monocyte activation in vitro. Furthermore, C0C1f induces strong upregulation of pro-inflammatory cytokines (interleukin-6 (IL-6), tumor necrosis factor α (TNFα), and interleukin-1β (IL-1β)) in cultured murine macrophages and human monocytes, resulting in a pro-inflammatory phenotype. We identified the toll-like receptor 4 (TLR4), toll-like receptor 2 (TLR2), and Advanced Glycosylation End Product-Specific Receptor (RAGE) as potential receptors for C0C1f whose activation leads to mobilization of the NFκB signaling pathway, a central mediator of the pro-inflammatory signaling cascade. Thus, C0C1f appears to be a key player in the initiation of inflammatory processes and might also play an important role upon MI.
Keywords: C0C1f; Cardiac myosin binding protein-C; Cell signaling/signal transduction; Inflammation; Ischemic biology - basic studies; Myocardial infarction.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
On behalf of all other authors, the corresponding author states that there is no conflict of interest
Figures


Similar articles
-
Vibrio cholerae porin OmpU mediates M1-polarization of macrophages/monocytes via TLR1/TLR2 activation.Immunobiology. 2015 Nov;220(11):1199-209. doi: 10.1016/j.imbio.2015.06.009. Epub 2015 Jun 5. Immunobiology. 2015. PMID: 26093918
-
Aloe vera downregulates LPS-induced inflammatory cytokine production and expression of NLRP3 inflammasome in human macrophages.Mol Immunol. 2013 Dec;56(4):471-9. doi: 10.1016/j.molimm.2013.05.005. Epub 2013 Aug 1. Mol Immunol. 2013. PMID: 23911403
-
Role of CD44 in Regulating TLR2 Activation of Human Macrophages and Downstream Expression of Proinflammatory Cytokines.J Immunol. 2018 Jan 15;200(2):758-767. doi: 10.4049/jimmunol.1700713. Epub 2017 Dec 1. J Immunol. 2018. PMID: 29196459 Free PMC article.
-
Surviving the infarct: A profile of cardiac myosin binding protein-C pathogenicity, diagnostic utility, and proteomics in the ischemic myocardium.Proteomics Clin Appl. 2014 Aug;8(7-8):569-77. doi: 10.1002/prca.201400011. Epub 2014 Jul 14. Proteomics Clin Appl. 2014. PMID: 24888514 Free PMC article. Review.
-
The Immune Function of Ly6Chi Inflammatory Monocytes During Infection and Inflammation.Curr Mol Med. 2017;17(1):4-12. doi: 10.2174/1566524017666170220102732. Curr Mol Med. 2017. PMID: 28231755 Review.
Cited by
-
The C0-C1f Region of Cardiac Myosin Binding Protein-C Induces Pro-Inflammatory Responses in Fibroblasts via TLR4 Signaling.Cells. 2021 May 26;10(6):1326. doi: 10.3390/cells10061326. Cells. 2021. PMID: 34073556 Free PMC article.
-
Role of the immune system in cardiac tissue damage and repair following myocardial infarction.Inflamm Res. 2017 Sep;66(9):739-751. doi: 10.1007/s00011-017-1060-4. Epub 2017 Jun 9. Inflamm Res. 2017. PMID: 28600668 Review.
-
When Innate Immunity Meets Angiogenesis-The Role of Toll-Like Receptors in Endothelial Cells and Pulmonary Hypertension.Front Med (Lausanne). 2020 Jul 31;7:352. doi: 10.3389/fmed.2020.00352. eCollection 2020. Front Med (Lausanne). 2020. PMID: 32850883 Free PMC article. Review.
-
Amino terminus of cardiac myosin binding protein-C regulates cardiac contractility.J Mol Cell Cardiol. 2021 Jul;156:33-44. doi: 10.1016/j.yjmcc.2021.03.009. Epub 2021 Mar 26. J Mol Cell Cardiol. 2021. PMID: 33781820 Free PMC article.
-
Chronic Benzene Exposure Aggravates Pressure Overload-Induced Cardiac Dysfunction.Toxicol Sci. 2021 Dec 28;185(1):64-76. doi: 10.1093/toxsci/kfab125. Toxicol Sci. 2021. PMID: 34718823 Free PMC article.
References
-
- Troidl C, Jung G, Troidl K, Hoffmann J, Mollmann H, Nef H, et al. The temporal and spatial distribution of macrophage subpopulations during arteriogenesis. Curr Vasc Pharmacol. 2013;11:5–12. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

