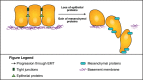
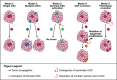
Cancer stem cells: Role in tumor growth, recurrence, metastasis, and treatment resistance
- PMID: 27611935
- PMCID: PMC5599212
- DOI: 10.1097/MD.0000000000004766
Cancer stem cells: Role in tumor growth, recurrence, metastasis, and treatment resistance
Abstract
Cancer stem cells (CSCs) are a class of pluripotent cells that have been observed in most types of solid and hematologic cancers. CSCs have been shown in numerous cancer models to be involved in tumor development, cell proliferation, and metastatic dissemination, while possessing a capacity for sustained self-renewal. CSCs, which typically represent a small proportion of total cells of a given tumor, also exhibit resistance to chemotherapy and radiotherapy. Indeed, exposure to these treatments may promote "stemness" in nonstem cancer cells, which may explain why successful therapeutic reduction of tumor bulk will often fail to produce clinical improvement. Acquisition of stemness involves epithelial-mesenchymal transition (EMT), in which epithelial cells are transformed into a mesenchymal phenotype characterized by increased capacities for migration, invasiveness, and resistance to apoptosis. EMT may also contribute to metastasis by driving dissemination of mesenchymal CSCs to distant locations, whereupon the CSCs revert to an epithelial phenotype to support metastatic tumor growth. Several different approaches to treatment aimed at overcoming the intrinsic resistance of CSCs to conventional therapies are currently being developed. These include agents targeting tumorigenic pathways, such as JAK2/STAT3 and PI3K/mTOR, and immunotherapies, including vaccines and natural killer cells employed to induce a T cell response.
Conflict of interest statement
The author reports no conflicts of interest.
Figures
Similar articles
-
Targeting microRNAs in epithelial-to-mesenchymal transition-induced cancer stem cells: therapeutic approaches in cancer.Expert Opin Ther Targets. 2015 Feb;19(2):285-97. doi: 10.1517/14728222.2014.975794. Epub 2015 Jan 7. Expert Opin Ther Targets. 2015. PMID: 25563894 Review.
-
Role of JAK/STAT3 Signaling in the Regulation of Metastasis, the Transition of Cancer Stem Cells, and Chemoresistance of Cancer by Epithelial-Mesenchymal Transition.Cells. 2020 Jan 15;9(1):217. doi: 10.3390/cells9010217. Cells. 2020. PMID: 31952344 Free PMC article. Review.
-
The Role of Cancer Stem Cells in Recurrent and Drug-Resistant Lung Cancer.Adv Exp Med Biol. 2016;890:57-74. doi: 10.1007/978-3-319-24932-2_4. Adv Exp Med Biol. 2016. PMID: 26703799 Review.
-
Cancer Stem Cells: Acquisition, Characteristics, Therapeutic Implications, Targeting Strategies and Future Prospects.Stem Cell Rev Rep. 2019 Jun;15(3):331-355. doi: 10.1007/s12015-019-09887-2. Stem Cell Rev Rep. 2019. PMID: 30993589 Review.
-
ZnAs@SiO2 nanoparticles as a potential anti-tumor drug for targeting stemness and epithelial-mesenchymal transition in hepatocellular carcinoma via SHP-1/JAK2/STAT3 signaling.Theranostics. 2019 Jun 9;9(15):4391-4408. doi: 10.7150/thno.32462. eCollection 2019. Theranostics. 2019. PMID: 31285768 Free PMC article.
Cited by
-
MAGE-A3 regulates tumor stemness in gastric cancer through the PI3K/AKT pathway.Aging (Albany NY). 2022 Nov 8;14(23):9579-9598. doi: 10.18632/aging.204373. Epub 2022 Nov 8. Aging (Albany NY). 2022. PMID: 36367777 Free PMC article.
-
Construction and Validation of a Prognostic Model Based on mRNAsi-Related Genes in Breast Cancer.Comput Math Methods Med. 2022 Oct 11;2022:6532591. doi: 10.1155/2022/6532591. eCollection 2022. Comput Math Methods Med. 2022. PMID: 36267313 Free PMC article.
-
GluOC promotes proliferation and metastasis of TNBC through the ROCK1 signaling pathway.Cancer Cell Int. 2024 Jul 25;24(1):263. doi: 10.1186/s12935-024-03445-8. Cancer Cell Int. 2024. PMID: 39054484 Free PMC article.
-
Elucidating the molecular signaling pathways of WAVE3.Ann Transl Med. 2020 Jul;8(14):900. doi: 10.21037/atm.2020.02.16. Ann Transl Med. 2020. PMID: 32793744 Free PMC article. Review.
-
Targeting hypoxia-induced tumor stemness by activating pathogen-induced stem cell niche defense.Front Immunol. 2022 Sep 29;13:933329. doi: 10.3389/fimmu.2022.933329. eCollection 2022. Front Immunol. 2022. PMID: 36248858 Free PMC article.
References
-
- Ajani JA, Song S, Hochster HS, et al. Cancer stem cells: the promise and the potential. Semin Oncol 2015; 42 (suppl 1):S3–S17. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous