Accelerated wound healing in a diabetic rat model using decellularized dermal matrix and human umbilical cord perivascular cells
- PMID: 27591919
- PMCID: PMC5069185
- DOI: 10.1016/j.actbio.2016.08.053
Accelerated wound healing in a diabetic rat model using decellularized dermal matrix and human umbilical cord perivascular cells
Abstract
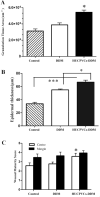
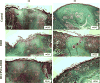
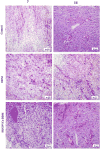
There is an unmet clinical need for novel wound healing strategies to treat full thickness skin defects, especially in diabetic patients. We hypothesized that a scaffold could perform dual roles of a biomechanical support and a favorable biochemical environment for stem cells. Human umbilical cord perivascular cells (HUCPVCs) have been recently reported as a type of mesenchymal stem cell that can accelerate early wound healing in skin defects. However, there are only a limited number of studies that have incorporated these cells into natural scaffolds for dermal tissue engineering. The aim of the present study was to promote angiogenesis and accelerate wound healing by using HUCPVCs and decellularized dermal matrix (DDM) in a rat model of diabetic wounds. The DDM scaffolds were prepared from harvested human skin samples and histological, ultrastructural, molecular and mechanical assessments were carried out. In comparison with the control (without any treatment) and DDM alone group, full thickness excisional wounds treated with HUCPVCs-loaded DDM scaffolds demonstrated an accelerated wound closure rate, faster re-epithelization, more granulation tissue formation and decreased collagen deposition. Furthermore, immunofluorescence analysis showed that the VEGFR-2 expression and vascular density in the HUCPVCs-loaded DDM scaffold treated group were also significantly higher than the other groups at 7days post implantation. Since the rates of angiogenesis, re-epithelization and formation of granulation tissue are directly correlated with full thickness wound healing in patients, the proposed HUCPVCs-loaded DDM scaffolds may fulfil a role neglected by current treatment strategies. This pre-clinical proof-of-concept study warrants further clinical evaluation.
Statement of significance: The aim of the present study was to design a novel tissue-engineered system to promote angiogenesis, re-epithelization and granulation of skin tissue using human umbilical cord perivascular stem cells and decellularized dermal matrix natural scaffolds in rat diabetic wound models. The authors of this research article have been working on stem cells and tissue engineering scaffolds for years. According to our knowledge, there is a lack of an efficient system for the treatment of skin defects using tissue engineering strategy. Since the rates of angiogenesis, re-epithelization and granulation tissue are directly correlated with full thickness wound healing, the proposed HUCPVCs-loaded DDM scaffolds perfectly fills the niche neglected by current treatment strategies. This pre-clinical study demonstrates the proof-of-concept that necessitates clinical evaluations.
Keywords: Decellularization; Dermal tissue engineering; Scaffold; Stem cell; Wound healing.
Copyright © 2016. Published by Elsevier Ltd.
Figures



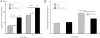

Similar articles
-
Nanofiber-acellular dermal matrix as a bilayer scaffold containing mesenchymal stem cell for healing of full-thickness skin wounds.Cell Tissue Res. 2019 Mar;375(3):709-721. doi: 10.1007/s00441-018-2927-6. Epub 2018 Oct 18. Cell Tissue Res. 2019. PMID: 30338376
-
Dynamic multiphoton imaging of acellular dermal matrix scaffolds seeded with mesenchymal stem cells in diabetic wound healing.J Biophotonics. 2018 Jul;11(7):e201700336. doi: 10.1002/jbio.201700336. Epub 2018 May 6. J Biophotonics. 2018. PMID: 29575792
-
[Influence of porcine urinary bladder matrix and porcine acellular dermal matrix on wound healing of full-thickness skin defect in diabetic mice].Zhonghua Shao Shang Za Zhi. 2020 Dec 20;36(12):1130-1138. doi: 10.3760/cma.j.cn501120-20200901-00399. Zhonghua Shao Shang Za Zhi. 2020. PMID: 33379849 Chinese.
-
Cutaneous wound healing in aging small mammals: a systematic review.Wound Repair Regen. 2015 May-Jun;23(3):318-39. doi: 10.1111/wrr.12290. Wound Repair Regen. 2015. PMID: 25817246 Review.
-
Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering.Artif Cells Nanomed Biotechnol. 2018 Jun;46(4):691-705. doi: 10.1080/21691401.2017.1349778. Epub 2017 Jul 12. Artif Cells Nanomed Biotechnol. 2018. PMID: 28697631 Review.
Cited by
-
Nanotechnology in Wound Care: One Step Closer to the Clinic.Mol Ther. 2018 Sep 5;26(9):2085-2086. doi: 10.1016/j.ymthe.2018.08.008. Epub 2018 Aug 16. Mol Ther. 2018. PMID: 30121229 Free PMC article. No abstract available.
-
Research progress in decellularized extracellular matrix-derived hydrogels.Regen Ther. 2021 May 18;18:88-96. doi: 10.1016/j.reth.2021.04.002. eCollection 2021 Dec. Regen Ther. 2021. PMID: 34095366 Free PMC article. Review.
-
Engraftment of bioengineered three-dimensional scaffold from human amniotic membrane-derived extracellular matrix accelerates ischemic diabetic wound healing.Arch Dermatol Res. 2021 Sep;313(7):567-582. doi: 10.1007/s00403-020-02137-3. Epub 2020 Sep 17. Arch Dermatol Res. 2021. PMID: 32940766
-
In vitro Differentiation of Hair Follicle Stem Cell into Keratinocyte by Simvastatin.Iran Biomed J. 2019 Nov;23(6):404-11. doi: 10.29252/ibj.23.6.404. Epub 2019 May 20. Iran Biomed J. 2019. PMID: 31104417 Free PMC article.
-
Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering.Bioact Mater. 2021 Sep 23;10:15-31. doi: 10.1016/j.bioactmat.2021.09.014. eCollection 2022 Apr. Bioact Mater. 2021. PMID: 34901526 Free PMC article. Review.
References
-
- Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997;276:75–81. - PubMed
-
- Gough NR. Reconstituting angiogenesis in vitro. Sci Signal. 2013;6:273.
-
- Gainza G, Pastor M, Aguirre JJ, Villullas S, Pedraz JL, Hernandez RM, Igartua M. A novel strategy for the treatment of chronic wounds based on the topical administration of rhEGF-loaded lipid nanoparticles: in vitro bioactivity and in vivo effectiveness in healing-impaired db/db mice. J Control Release. 2014;185:51–61. - PubMed
-
- Bremer AA. MnSOD, angiogenesis, and wounds: let the healing begin. Sci Transl Med. 2010;2(59):59–181.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

