The ATM Kinase Restrains Joining of Both VDJ Signal and Coding Ends
- PMID: 27574300
- PMCID: PMC5101155
- DOI: 10.4049/jimmunol.1600597
The ATM Kinase Restrains Joining of Both VDJ Signal and Coding Ends
Abstract
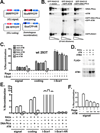
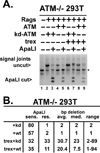
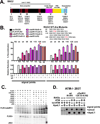
The evidence that ATM affects resolution of RAG-induced DNA double-strand breaks is profuse and unequivocal; moreover, it is clear that the RAG complex itself cooperates (in an undetermined way) with ATM to facilitate repair of these double-strand breaks by the classical nonhomologous end-joining pathway. The mechanistic basis for the cooperation between ATM and the RAG complex has not been defined, although proposed models invoke ATM and RAG2's C terminus in maintaining the RAG postcleavage complex. In this study, we show that ATM reduces the rate of both coding and signal joining in a robust episomal assay; we suggest that this is the result of increased stability of the postcleavage complex. ATM's ability to inhibit VDJ joining requires its enzymatic activity. The noncore C termini of both RAG1 and RAG2 are also required for ATM's capacity to limit signal (but not coding) joining. Moreover, potential phosphorylation targets within the C terminus of RAG2 are also required for ATM's capacity to limit signal joining. These data suggest a model whereby the RAG signal end complex is stabilized by phosphorylation of RAG2 by ATM.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures

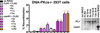
Similar articles
-
Functional intersection of ATM and DNA-dependent protein kinase catalytic subunit in coding end joining during V(D)J recombination.Mol Cell Biol. 2013 Sep;33(18):3568-79. doi: 10.1128/MCB.00308-13. Epub 2013 Jul 8. Mol Cell Biol. 2013. PMID: 23836881 Free PMC article.
-
Repair of chromosomal RAG-mediated DNA breaks by mutant RAG proteins lacking phosphatidylinositol 3-like kinase consensus phosphorylation sites.J Immunol. 2011 Aug 15;187(4):1826-34. doi: 10.4049/jimmunol.1101388. Epub 2011 Jul 8. J Immunol. 2011. PMID: 21742970 Free PMC article.
-
Nemo-Dependent, ATM-Mediated Signals from RAG DNA Breaks at Igk Feedback Inhibit V κ Recombination to Enforce Igκ Allelic Exclusion.J Immunol. 2022 Jan 15;208(2):371-383. doi: 10.4049/jimmunol.2100696. Epub 2021 Dec 29. J Immunol. 2022. PMID: 34965965 Free PMC article.
-
The influence of heterochromatin on DNA double strand break repair: Getting the strong, silent type to relax.DNA Repair (Amst). 2010 Dec 10;9(12):1273-82. doi: 10.1016/j.dnarep.2010.09.013. Epub 2010 Oct 30. DNA Repair (Amst). 2010. PMID: 21036673 Review.
-
The RAG proteins and V(D)J recombination: complexes, ends, and transposition.Annu Rev Immunol. 2000;18:495-527. doi: 10.1146/annurev.immunol.18.1.495. Annu Rev Immunol. 2000. PMID: 10837067 Review.
Cited by
-
Activation of DNA-PK by hairpinned DNA ends reveals a stepwise mechanism of kinase activation.Nucleic Acids Res. 2020 Sep 18;48(16):9098-9108. doi: 10.1093/nar/gkaa614. Nucleic Acids Res. 2020. PMID: 32716029 Free PMC article.
-
Loss of H3K36 Methyltransferase SETD2 Impairs V(D)J Recombination during Lymphoid Development.iScience. 2020 Mar 27;23(3):100941. doi: 10.1016/j.isci.2020.100941. Epub 2020 Feb 27. iScience. 2020. PMID: 32169821 Free PMC article.
-
The Conserved ATM Kinase RAG2-S365 Phosphorylation Site Limits Cleavage Events in Individual Cells Independent of Any Repair Defect.Cell Rep. 2017 Oct 24;21(4):979-993. doi: 10.1016/j.celrep.2017.09.084. Cell Rep. 2017. PMID: 29069605 Free PMC article.
-
Deciphering phenotypic variance in different models of DNA-PKcs deficiency.DNA Repair (Amst). 2019 Jan;73:7-16. doi: 10.1016/j.dnarep.2018.10.004. Epub 2018 Oct 30. DNA Repair (Amst). 2019. PMID: 30409670 Free PMC article.
-
Full length RAG2 expression enhances the DNA damage response in pre-B cells.Immunobiology. 2021 May;226(3):152089. doi: 10.1016/j.imbio.2021.152089. Epub 2021 Apr 6. Immunobiology. 2021. PMID: 33873062 Free PMC article.
References
-
- Lieber MR, Yu K, Raghavan SC. Roles of nonhomologous DNA end joining, V(D)J recombination, and class switch recombination in chromosomal translocations. DNA repair. 2006;5:1234–1245. - PubMed
-
- Gellert M. V(D)J recombination: RAG proteins, repair factors, and regulation. Annual review of biochemistry. 2002;71:101–132. - PubMed
-
- Hesse JE, Lieber MR, Mizuuchi K, Gellert M. V(D)J recombination: a functional definition of the joining signals. Genes & development. 1989;3:1053–1061. - PubMed
-
- van Gent DC, Ramsden DA, Gellert M. The RAG1 and RAG2 proteins establish the 12/23 rule in V(D)J recombination. Cell. 1996;85:107–113. - PubMed
-
- Hiom K, Gellert M. A stable RAG1-RAG2-DNA complex that is active in V(D)J cleavage. Cell. 1997;88:65–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

