Oxidant Sensing by TRPM2 Inhibits Neutrophil Migration and Mitigates Inflammation
- PMID: 27569419
- PMCID: PMC5455786
- DOI: 10.1016/j.devcel.2016.07.014
Oxidant Sensing by TRPM2 Inhibits Neutrophil Migration and Mitigates Inflammation
Abstract
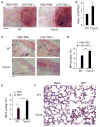
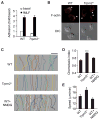
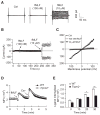
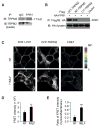
Blood neutrophils perform an essential host-defense function by directly migrating to bacterial invasion sites to kill bacteria. The mechanisms mediating the transition from the migratory to bactericidal phenotype remain elusive. Here, we demonstrate that TRPM2, a trp superfamily member, senses neutrophil-generated reactive oxygen species and restrains neutrophil migration. The inhibitory function of oxidant sensing by TRPM2 requires the oxidation of Cys549, which then induces TRMP2 binding to formyl peptide receptor 1 (FPR1) and subsequent FPR1 internalization and signaling inhibition. The oxidant sensing-induced termination of neutrophil migration at the site of infection permits a smooth transition to the subsequent microbial killing phase.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
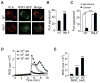
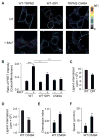
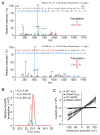
Comment in
-
A Radical Break: Restraining Neutrophil Migration.Dev Cell. 2016 Sep 12;38(5):448-50. doi: 10.1016/j.devcel.2016.08.017. Dev Cell. 2016. PMID: 27623379
Similar articles
-
A Radical Break: Restraining Neutrophil Migration.Dev Cell. 2016 Sep 12;38(5):448-50. doi: 10.1016/j.devcel.2016.08.017. Dev Cell. 2016. PMID: 27623379
-
Propofol inhibits endogenous formyl peptide-induced neutrophil activation and alleviates lung injury.Free Radic Biol Med. 2018 Dec;129:372-382. doi: 10.1016/j.freeradbiomed.2018.09.048. Epub 2018 Oct 10. Free Radic Biol Med. 2018. PMID: 30312762
-
Novel role of reactive oxygen species-activated Trp melastatin channel-2 in mediating angiogenesis and postischemic neovascularization.Arterioscler Thromb Vasc Biol. 2015 Apr;35(4):877-87. doi: 10.1161/ATVBAHA.114.304802. Epub 2015 Feb 12. Arterioscler Thromb Vasc Biol. 2015. PMID: 25675998 Free PMC article.
-
TRPM2 channel regulates endothelial barrier function.Adv Exp Med Biol. 2010;661:155-67. doi: 10.1007/978-1-60761-500-2_10. Adv Exp Med Biol. 2010. PMID: 20204729 Review.
-
Chemical physiology of oxidative stress-activated TRPM2 and TRPC5 channels.Prog Biophys Mol Biol. 2010 Sep;103(1):18-27. doi: 10.1016/j.pbiomolbio.2010.05.005. Epub 2010 May 27. Prog Biophys Mol Biol. 2010. PMID: 20553742 Review.
Cited by
-
pH-Channeling in Cancer: How pH-Dependence of Cation Channels Shapes Cancer Pathophysiology.Cancers (Basel). 2020 Sep 2;12(9):2484. doi: 10.3390/cancers12092484. Cancers (Basel). 2020. PMID: 32887220 Free PMC article. Review.
-
TRPM channels in health and disease.Nat Rev Nephrol. 2024 Mar;20(3):175-187. doi: 10.1038/s41581-023-00777-y. Epub 2023 Oct 18. Nat Rev Nephrol. 2024. PMID: 37853091 Review.
-
The TRPM2 Ion Channel Regulates Inflammatory Functions of Neutrophils During Listeria monocytogenes Infection.Front Immunol. 2020 Feb 4;11:97. doi: 10.3389/fimmu.2020.00097. eCollection 2020. Front Immunol. 2020. PMID: 32117251 Free PMC article.
-
Neutrophil Activation of Endothelial Cell-Expressed TRPM2 Mediates Transendothelial Neutrophil Migration and Vascular Injury.Circ Res. 2017 Oct 13;121(9):1081-1091. doi: 10.1161/CIRCRESAHA.117.311747. Epub 2017 Aug 8. Circ Res. 2017. PMID: 28790198 Free PMC article.
-
In silico Identification of 10 Hub Genes and an miRNA-mRNA Regulatory Network in Acute Kawasaki Disease.Front Genet. 2021 Mar 25;12:585058. doi: 10.3389/fgene.2021.585058. eCollection 2021. Front Genet. 2021. PMID: 33868359 Free PMC article.
References
-
- Bender JG, McPhail LC, Van Epps DE. Exposure of human neutrophils to chemotactic factors potentiates activation of the respiratory burst enzyme. J Immunol. 1983;130:2316–2323. - PubMed
-
- Brozna JP. Shwartzman reaction. Semin Thromb Hemost. 1990;16:326–332. - PubMed
-
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

